Utilization of the carbon dioxide (CO2) captured from coal-fired power plants or other anthropogenic CO2 sources as the injection gas to enhance oil recovery (EOR) was thought to be feasible, economical, and urgent owing to the fast-increasing demand of CO2 for EOR purposes. It can reduce the
CO2
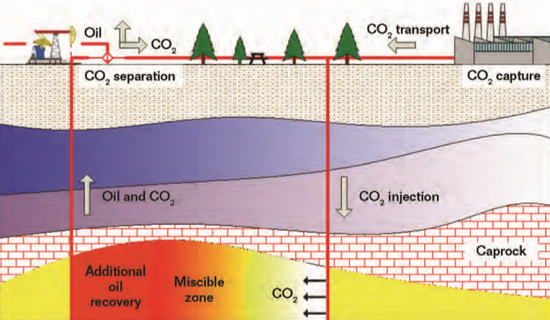
emission after its utilization in the EOR process, and this kind of technology is often referred to as carbon capture, utilization, and storage (CCUS), as depicted in Figure 1. There are various types of other CCUS systems, including enhanced coalbed methane recovery, enhanced geothermal systems, enhanced shale gas recovery, and enhanced gas recovery.1 CCUS can lower the total cost of carbon emission reduction compared to carbon capture and storage technology. Both technologies are thought to be effective ways for human beings to mitigate the global climate change problem before the large-scale application of renewable energy sources.
Typically, CO2 captured from coal-fired power plants inevitably contains a certain amount of impurities, such as SOx, NOx, hydrogen sulfide (H2S), O2, Ar, N2, H2, CO, and water (H2O), depending on the specific capture technology. This feature is rather different from the CO2 produced from the natural CO2 well, which usually contains high-purity CO2. If the impurities in anthropogenic CO2 are removed to an ultra-low impurity concentration, it will greatly increase the CCUS cost, one of the principal obstacles for large-scale applications of the technology. If impurities were not thoroughly removed, they may have a corrosive effect on CO2 pipelines2-5 and could also have chemical reactions with caprocks, which could possibly affect CO2 injectivity and storage safety.
In recent years, the lack of CO2 supplies from natural CO2 wells for EOR has resulted in a great need for the utilization of CO2 captured from anthropogenic CO2 sources, such as coal-fired power plants. In fact, small amounts of sulfur dioxide (SO2), O2, and NOx have been co-injected with CO2 into oil reservoirs for more than 30 years in North America,6 although the impurity concentration was not clear. In China, there are more than 10 CO2-EOR projects under planning or in service and several of them use CO2 captured from anthropogenic sources.1 However, a few of them have encountered operational issues, such as corrosion problems on well materials, which have to be solved before large-scale application.
When the anthropogenic CO2 was applied to the EOR system, the corrosion effects of impurities on the system still require comprehensive assessment, including the effects on the capture, transport, EOR, and permanent geological storage processes. The present work primarily addresses this matter and determined that the corrosion effects of the impurities in anthropogenic CO2 on the EOR-CCUS system were complex, unique, and non-negligible.
Corrosion Effects of Impurities in Capture Process
Alkanolamines are usually employed as absorbents in the CO2 capture process, and they are also well known for their usage in the oil and gas industries to remove acid gas impurities, such as removing CO2 and H2S from natural gas. During this kind of CO2 capture process, the impurities, such as O2, SOx, and NOx, can promote the degradation of alkanolamines in the CO2 scrubbing process, leading to the formation of heat-stable salts (HSS) that will remain in the amine solution.
The HSS and acid gas impurities themselves can also exacerbate the corrosion problems within the CO2 capture units. Although the precipitation of siderites on the carbon steel (CS) surface at the high-temperature regions can remarkably decrease corrosion rates,7-8 utilization of stainless steel at certain parts of the capture unit is essential in order to control corrosion. Recently, it was described that the presence of sulfurous acid (H2SO3), owing to the SO2 impurity, can accelerate the corrosion of a valve steel by promoting anodic dissolution and also by introducing a new cathodic reaction, the direct reduction of H2SO3 or bisulfite (HSO3–), into the cathodic process when coexisting with CO2.9 This acceleration effect may also exist in the CO2 capture process.The stress corrosion cracking of both alloys and CS may occur in the amine solution at higher temperature conditions. The presence of impurities such as oxygen and nitrates owing to the presence of NOx could accelerate this effect. Optimized material selection is needed to control this kind of material failure.
Corrosion Effects of Impurities in Transport Process
Typically, CO2 is compressed to the supercritical phase and transported by pipelines to the EOR and storage sites, a process that was thought to be more economical than other means, such as by truck, rail, and ship.10 When water and acid gas impurities (SO2, NOx, and H2S) coexist in the pipeline, the pipeline internal corrosion problem will occur because of the condensation or adsorption effect of water on the steel surface and the subsequent dissolution of corrosive species, even though the water concentration is under the solubility limit in supercritical CO2. The uniform corrosion rates of the pipeline steels in impure supercritical CO2 environments are always around several mm/y depending on specific conditions.
Meanwhile, pitting corrosion is more harmful to pipeline integrity; therefore, researchers are more curious regarding pitting corrosion in supercritical CO2 environments, especially its propagation ability. However, Sun, et al.11 recently reported that the propagation rate of corrosion pits of pipeline steel in the depth direction obviously decreased with time, and the corrosion type changed from localized corrosion to general corrosion with long-term exposure to a static, water-saturated supercritical CO2-SO2-NO2-H2S-O2 environment. These conclusions might be different when the steel was exposed to the water-rich phase under the same conditions, which might be encountered if there was a large amount of condensed water accumulated at the bottom of the pipeline. Attention must also be paid to the corrosion of compressor materials and the degradation of polymer materials owing to the impurities in CO2.
Therefore, a low concentration of water is required for almost all of the existing CO2 pipelines to control the corrosion problems. The presence of free water in the CO2 pipeline can also lead to the formation of hydrates at low-temperature conditions, which could block the pipeline and result in operational accidents. When making a CO2 quality recommendation for the CO2 pipeline transport, the impact of impurities on the health, safety, and environment must also be considered, including the impacts of CO, hydrogen cyanide (HCN), Hg, H2S, SOx, NOx, and amines.
Corrosion Effects of Impurities in EOR and Geological Storage Processes
When the CO2 mixtures are injected through the injection well, corrosion will not be a major problem based on the dry-out effect during the injection process. However, the presence of acid gases and formation water can cause the formation of strong acids, which will lead to severe corrosion on the well infrastructures, particularly on the production well. The corrosion phenomenon in production wells is complex, with impurities from the injected CO2 mixtures and mineral ions in the formation water mixed in the multiphase with crude oil, gas, water, and solid particles at the high-temperature, high-pressure conditions.
In 2015, there was a casing material failure accident in an oil field in China using CO2 mixtures as the EOR displacement gas, and the failure analysis results showed that there was 30 to 40 ppmv H2S impurity in the CO2 source, leading to sulfide stress cracking (SSC).12
Acidic gases can have chemical effects on the rocks. SOx can react with rocks to form sulfate that can be deposited, reduce rock porosity, and eventually diminish injectivity. When SO2 and H2S are co-sequestered, the deposition of elemental sulfur may also reduce injectivity. Nogueira and Mamora13 concluded that injection with no more than 1 mol% impurities would result in practically the same volume of CO2 being stored as injecting pure CO2, but it would have a lower separation cost compared to an extremely high-purity CO2 scenario.
The separation of CO2 and other impurities with oil production is costly, and the impurities may also influence downstream production. For example, O2 could promote the growth of aerobic bacteria, which induces biodegradation of crude oil and adversely affects oil recovery and refinement.14 This may also induce the microbiologically influenced corrosion.
When the injection process is terminated, the corrosion of well steels and cements will be problematic owing to the loss of the desiccation effect of the well zone during the injection process. The well integrity under long-term exposure might be a real challenge for the permanent geological storage of CO2. The leakage of CO2 from the wells is a major concern for the CCUS technology.
General Discussion
To control the corrosion problems of steels in CCUS systems, the utilization of inhibitors could be an economical and effective method, besides the application of corrosion-resistant alloys, coatings, and cathodic protection. However, when various acid gases are present, it seems to be hard to find inhibitors that can reduce the corrosion rates of steels under the attack of both strong and weak acids, including sulfuric acid (H2SO4), nitric acid (HNO3), H2SO3, carbonic acid (H2CO3), and H2S. Recently, it was reported that piperazine can neutralize the acidic gas impurities and inhibit the corrosion of CS in supercritical CO2-saturated aqueous phases containing SO2, nitrogen dioxide (NO2), and O2 impurities.15 Piperazine and its derivatives are potential inhibitors of steel corrosion in CCUS environments. Meanwhile, controlling the water content in the CO2 streams can be an alternative corrosion control strategy.
The safety of CCUS is one of the most important issues that impede its large-scale applications. Although acid gas impurities have a dissolution effect on caprocks, a second-phase deposition can increase the porosity as well as enhance the safety of CCUS. At geological fault locations, seismic activity and/or failure of well integrity could be a huge threat to CCUS safety, which can result in a fast leakage of sequestered CO2 to the earth surface and seriously pose risks to the safety of human beings. However, it is not probable that seismic activity is a problem at all CO2 storage sites at the same time. Nevertheless, there is a great necessity to choose CO2 storage sites far away from populated regions. The well integrity and the impact of potential activity resulting from CO2 storage are the greatest threat to CCUS safety.
Acknowledgments
The author would like to acknowledge that this work was financially supported by National Key R&D Program of China (2017YFC0805800), National Natural Science Foundation of China (51604289), Beijing Natural Science Foundation (2172048), and Science Foundation of China University of Petroleum, Beijing (2462014YJRC043).
References
1 H.J. Liu, P. Were, Q. Li, et al., “Worldwide Status of CCUS Technologies and their Development and Challenges in China,” Geofluids 2017, pp. 1-25.
2 Y. Xiang, M. Xu, Y.-S. Choi, “State-of-the-Art Overview of Pipeline Steel Corrosion in Impure Dense CO2 for CCS Transportation: Mechanisms and Models,” Corros. Eng. Sci. Techn. 52, 7 (2017): pp. 485-509.
3 Y. Hua, R. Barker, A. Neville, “Understanding the Influence of SO2 and O2 on the Corrosion of Carbon Steel in Water-Saturated Supercritical CO2,” Corrosion 71, 5 (2015): pp. 667683.
4 L. Wei, X. Pang, K. Gao, “Corrosion of Low Alloy Steel and Stainless Steel in Supercritical CO2/H2O/H2S Systems,” Corros. Sci. 111 (2016): pp. 637-648.
5 M. Xu, Q. Zhang, Z. Wang, et al., “Effect of High-Concentration O2 on Corrosion Behavior of X70 Steel in Water-Containing Supercritical CO2 with SO2,” Corrosion 73, 3 (2017): pp. 290-302.
6 J.J. Taber, “Fate of Small Concentrations of SO2, NOx and O2 When Injected with CO2 into Oil Reservoirs” (Lemont, IL: Argonne National Laboratories, 1985).
7 Y. Xiang, Y.-S. Choi, Y. Yang, et al., “Corrosion of Carbon Steel in MDEA-Based CO2 Capture Plants Under Regenerator Conditions: Effects of O2 and Heat-Stable Salts,” Corrosion 71, 1 (2015): pp. 30-37.
8 L. Zheng, J. Landon, N.S. Matin, et al., “FeCO3 Coating Process Toward the Corrosion Protection of Carbon Steel in a Postcombustion CO2 Capture System,” Ind. & Eng. Chem. Res. 55, 14 (2016): pp. 3,939-3,948.
9 Y. Xiang, C. Li, Z. Long, et al., “Electrochemical Behavior of Valve Steel in a CO2/Sulfurous Acid Solution,” Electrochim. Acta 258 (2017): pp. 909-918.
10 V.E. Onyebuchi, A. Kolios, D.P. Hanak, et al., “A Systematic Review of Key Challenges of CO2 Transport via Pipelines,” Renew. Sust. Energ. Rev. 81, Part 2 (2018): pp. 2,563-2,583.
11 C. Sun, J. Sun, Y. Wang, et al., “Effect of Impurity Interaction on the Corrosion Film Characteristics and Corrosion Morphology Evolution of X65 Steel in Water-Saturated Supercritical CO2 System,” Int. J. Greenh. Gas Con. 65, Supplement C (2017): pp. 117-127.
12 X. Zhao, Z. He, J. Liu, et al., “Research Status of CCUS Corrosion Control Technology,” Petrol. Tubular Goods & Instrum. 3, 3 (2017): pp. 1-6.
13 M. Nogueira, D.D. Mamora, “Effect of FlueGas Impurities on the Process of Injection and Storage of CO2 in Depleted Gas Reservoirs,” J. Energ. Resour-ASME 130, 1 (2008): pp. 013301-013305.
14 D.M. Jones, I.M. Head, N.D. Gray, et al., “Crude-Oil Biodegradation via Methanogenesis in Subsurface Petroleum Reservoirs,” Nature 451 (2008): pp. 176-180.
15 Y. Xiang, Z. Long, C. Li, et al., “Inhibition of N80 Steel Corrosion in Impure Supercritical CO2 and CO2-Saturated Aqueous Phases by Using Imino Inhibitors,” Int. J. Greenh. Gas Con. 63 (2017): pp. 141-149