Editor’s note:
Learn more about the
Flint, Michigan drinking water crisis caused by corrosion in this new materialsperformance.com
quarterly special feature, The Science Behind It. Read the following MP article to get the basic facts, and
then explore the science behind the corrosion problem, which is presented in
several related CORROSION articles
listed at the end of the article.
Over the past year, overall awareness of the issue of lead contamination of potable water has increased due to the high levels of lead detected in drinking water from homes in Flint, Michigan in 2015. The cause of lead leaching into the water in Flint was corrosion of the water distribution system’s service pipes and solder. This was determined by a team of research scientists and students from Virginia Polytechnic Institute and State University (Virginia Tech) (Blacksburg, Virginia) that worked with Flint residents to study the water and establish the cause of the widespread contamination.
In a water distribution system, corrosion of pipes and solder causes the dissolution of these materials. The metals then leach into the water supply, which causes lead and copper concentrations in the water to increase.
According to the U.S. Environmental Protection Agency (EPA), it was common practice up through the early 1900s to use lead pipes for interior plumbing and the service connections that join residences to public water supplies. Homes built before 1986 are more likely to have lead pipes, fixtures, and solder that can leach significant amounts of lead into the tap water, especially hot water. Although copper pipes have replaced lead pipes in most residential plumbing, the use of lead solder with copper pipes had been widespread and was regarded as the major cause of lead contamination in U.S. households in the 1990s. The amount of lead that contaminates drinking water is influenced by several factors, such as the corrosivity of the water, amount of lead it contacts, and the amount of wear in the pipes; the length of time that the water stays in the pipes; and the presence of protective scales or film inside plumbing components. Water’s corrosivity is determined by characteristics such as low pH (acidity), dissolved oxygen, and low mineral content.
Because lead contamination of drinking water often results from corrosion of the plumbing materials belonging to water system customers, the EPA established a treatment technique regulation for lead known as the Lead and Copper Rule (LCR). This rule requires water treatment facilities to control the corrosivity of drinking water and make it less corrosive to the materials it contacts on its way to consumers’ faucets. Adjusting alkalinity and pH is one technique many water distribution systems use for corrosion control. Phosphate-based corrosion inhibitors also have been widely used to control lead and copper release. Phosphate-based corrosion inhibitors are chemicals that have orthophosphate in their formulation. Orthophosphate bonds with lead and copper ions to form a protective coating that has a strong tendency to stay in solid form and not dissolve in water.1
A Change in Flint’s Water Source
The City of Flint had signed a long-term water supply contract with the Detroit Water and Sewerage Department (DWSD) and utilized this water source for almost 50 years. The DWSD water supply had been treated for corrosion control with orthophosphate for over 20 years and was on a maintenance dose of orthophosphate since its corrosion control treatment was fully optimized. Although Flint had a water treatment plant, it served as an emergency backup; it was upgraded periodically to keep it ready for use and was put into operation four times a year to maintain its readiness.
In April 2014, because of annual rate increases from the DWSD, the Flint City Council voted to join the Karegnondi Water Authority (KWA), which would be developing a raw water supply pipeline from Lake Huron. The water supply contract with DWSD was subsequently terminated, and the Flint water treatment plant began treating water from the Flint River on a full-time basis and distributing the treated water to residents and other customers. When the plant went into full-time operation, the Michigan Department of Environmental Quality (MDEQ) did not implement corrosion control, as mandated by the LCR. Instead, the Flint water treatment plant was allowed to complete two six-month monitoring periods without corrosion control and then the MDEQ would decide if corrosion control treatment was necessary.
Not too long after the city began distributing treated river water from its own water treatment plant, problems were noted concerning the water’s odor, taste, and appearance. According to the Virginia Tech researchers, discolored water can be caused by metallic rust that is released into the water from unlined cast iron pipes or iron service lines that have corrosion occurring along their lengths. Additionally, microorganisms may be living in the corroded pipes, such as sulfate-reducing bacteria (SRB) that produce hydrogen sulfide (H2S) and its characteristic rotten-egg smell, which contribute to the acidity of the water and increase its corrosivity.2 To prevent the growth of microorganisms, chlorine is added to the water as a disinfectant, and maintaining a residual amount of chlorine in the water protects public health against the presence of opportunistic pathogens (OPs)—microbes capable of causing disease when a host has lowered resistance. Since chlorine is consumed by iron corrosion, chlorine added to the water going through corroding pipes will quickly disappear, making it even more likely that harmful bacteria are growing in the water pipes.
When the first round of Flint’s water quality monitoring ended in December 2014, the Flint water treatment plant’s exemption from corrosion control treatment was rescinded due to the high lead levels found in tap water, and it was required to implement corrosion control treatment. Several months later, in February 2015, the EPA was contacted by a Flint resident about the high levels of lead found in the home’s drinking water (the EPA lead action level is 15 ppb and the home’s water tested at 104 ppb in February and 397 ppb when tested again the next month). The Flint water treatment plant had not implemented the required optimized corrosion control treatment.
Virginia Tech Researchers Study the Water
In August 2015, the Virginia Tech researchers, led by Marc Edwards, the Charles Lunsford Professor in the university’s Department of Civil and Environmental Engineering, traveled to Flint to sample and test the water and evaluate the water quality. They sampled water at sites used by the city for its water distribution system monitoring, and analyzed the samples for OPs, water temperature, dissolved oxygen, and chlorine residual. The researchers also sampled tap water at four businesses that were still using water from the DWSD, four homes that used the city’s treated water from the Flint River, and two homes where residents had reported health issues. Chlorine levels were also monitored at one site that used the DWSD water and another site that used the city’s treated water. Testing results did not indicate the presence of OPs in the Flint city-treated water; however, chlorine residuals were low throughout the system.

At their Virginia Tech lab, the team conducted lead testing on 252 water samples provided by Flint residents. Out of 252 water samples taken from Flint homes, 101 (40.1%) had first draw lead levels >5 ppb and 42 samples (16.7%)
 exceeded the 15 ppb lead action level. According to the researchers, Flint’s 90th percentile lead value was 25 ppb in their survey. Several samples exceeded 100 ppb, and one sample collected after 45 seconds of water flushing exceeded 1,000 ppb.3
exceeded the 15 ppb lead action level. According to the researchers, Flint’s 90th percentile lead value was 25 ppb in their survey. Several samples exceeded 100 ppb, and one sample collected after 45 seconds of water flushing exceeded 1,000 ppb.3
The chemistry of Flint River water was known to be highly corrosive to lead plumbing as well as iron pipe due to its high chloride content, which was about eight times higher than the chloride content in the DWSD water. During their visit in Flint, the researchers tested copper pipe pieces joined with lead solder that was 50% lead by weight to test the relative corrosivity of Flint’s treated river water vs. the DWSD water. (Although lead solder was banned in 1986 for use in drinking water systems, almost all the homes in Flint were built before then and plumbing pipes contained lead solder.) For the test, they put the a copper piece with lead solder in a container filled with either DWSD water; Flint city-treated water; or Flint city-treated water with orthophosphate added to determine whether the corrosion inhibitor would stop lead corrosion.
During the experiment, the Flint city-treated waters (with and without orthophosphate) started turning white due to rapid corrosion of the lead, while the DWSD water remained clear. The researchers then took water from each container after one, two, and three weeks and measured the lead levels in the water. After three weeks, the test results showed lead levels in Flint city-treated water without the corrosion inhibitor were slightly above hazardous waste levels (5,000 ppb), and 19 times higher than the lead levels detected in the DWSD water.
 The lead levels in the Flint city-treated water with a corrosion inhibitor eventually dropped to low levels. The researchers concluded that the corrosivity of the Flint city-treated water caused more lead from plumbing to leach into the water compared to the DWSD water, and that the water corrosivity of Flint River would have been countered by the addition of orthophosphate.
The lead levels in the Flint city-treated water with a corrosion inhibitor eventually dropped to low levels. The researchers concluded that the corrosivity of the Flint city-treated water caused more lead from plumbing to leach into the water compared to the DWSD water, and that the water corrosivity of Flint River would have been countered by the addition of orthophosphate.
Edwards notes that iron corrosion is also a major concern and could potentially be the most expensive problem facing Flint and other water utilities. The researchers decided to establish the rate of iron corrosion when exposed to the Flint city-treated water without a corrosion inhibitor treatment.4
Tests were conducted over six days to determine the decay of chlorine over time in the Flint city-treated water compared to the DWSD water. The researchers demonstrated that rapid iron corrosion in the Flint city-treated water was consuming the chlorine disinfectant and causing much more iron release than the DWSD water. They expanded their tests by exposing a steel sample (a 99.99% iron nail) to DWSD water, Flint city-treated water, and Flint city-treated water with orthophosphate added. The water contacting the steel was changed every Monday, Wednesday, and Friday.
After a month, the iron samples were photographed and weight loss was measured. The nail exposed to Flint city-treated water exhibited much more surface rust than the nail exposed to the DWSD water. The weight loss measurements of the nails were then used to determine the corrosion rate.

For the nail in Flint city-treated water, the corrosion rate was 8.6 times higher than it was for the nail in DWSD water. Even with the added corrosion inhibitor in the Flint city-treated water, the corrosion rate was still 3.5 times higher than in the DWSD water.
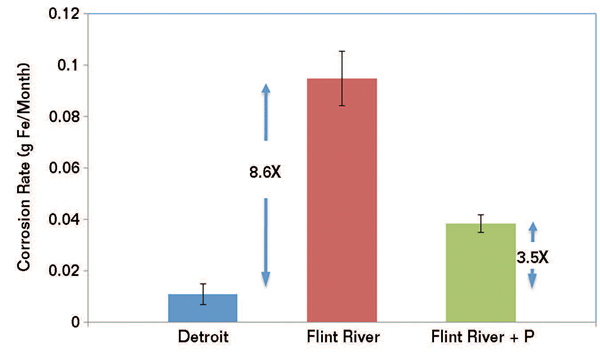
Metal pipe corrosion can be further accelerated by microbiologically influenced corrosion (MIC), Edwards comments. Using Biological Activity Reaction Test (BART) kits, the researchers tested the Flint city-treated water for specific MIC-causing bacteria including heterotrophic aerobic bacteria (HAB), acid-producing bacteria (APB), iron-reducing bacteria (IRB), SRB, and slime forming bacteria (SLYM). They found all of these corrosion-causing bacteria in the Flint city-treated water. According to Edwards, this was probably due to low chlorine, but might also be due to organic matter in the Flint city-treated water, which would provide a food source for the bacteria. When the researchers tested samples of DWSD water, the levels of corrosion-causing bacteria were much lower.
Flint Switches Back to Less Corrosive Water
On October 16, 2015, the City of Flint switched back to DWSD as the source for the city’s drinking water. Because Flint had been using city-treated water from the Flint River for 18 months without corrosion control treatment, the protective scaling on pipes and plumbing formed by the orthophosphate corrosion inhibitor in the DWSD water had been destroyed by the more corrosive water. Discussions between the MDEQ and EPA were held to determine the best course of action for obtaining optimal corrosion control treatment for the water purchased from DWSD. They decided to add a supplemental dose of orthophosphate to the water to enhance the pipe passivation in Flint since DWSD used a maintenance dose of orthophosphate in the water.
The orthophosphate dose was increased so the distribution system’s phosphate residual was a minimum of 3.1 mg/L.5 The corrosion control optimization plan also called for a minimum pH of 7.0 to be maintained. Additionally, the EPA and the MDEQ recommended that Flint residents run the water in their homes and businesses to help distribute the orthophosphate in the drinking water system and build-up the protective coating in the pipes. The EPA noted that attention to building a protective coating in pipes does not mean there is any less urgency to remove lead service lines.6
Bibliography
“Flint Water Advisory Task Force—Final Report.” March 2016. www.michigan.gov/documents/ snyder/FWATF_FINAL_REPORT_ 21March2016_517805_7.pdf. May 17, 2016
References
1 “Optimal Corrosion Control Treatment Evaluation Technical Recommendations for Primacy Agencies and Public Water Systems,” EPA, 816-B-16-003, March 2016.
2 N. Zhu, S. Roy, “The Unintended Consequences of Migrating to Flint River Water,” Flint Water Study Updates, Aug. 23, 2015, http://flintwaterstudy.org/2015/08/the-unintended-consequences-of-migrating-to-flint-river-water/ (May 17, 2016).
3 “Lead testing results for water sampled by residents,” Flint Water Study Updates, http://flintwaterstudy.org/information-for-flint-residents/results-for-citizen-testing-for-lead-300-kits/ (May 17, 2016).
4 M. Edwards, “Research Update: Corrosivity of Flint Water to Iron Pipes in the City—A Costly Problem,” Flint Water Study Updates, http://flintwaterstudy.org/2015/09/research-update-corrosivity-of-flint-water-to-iron-pipes-in-the-city-a-costly-problem (May 17, 2016).
5 “Plan for Optimization of Corrosion Control,” MDEQ, Taking Action on Flint Water, http://www.michigan.gov/documents/flintwater/Plan_for_Optimization_of_Corrosion_Control_514633_7.pdf (May 17, 2016).
6 “EPA, DEQ and City of Flint Recommend Flushing Water to Speed Recovery of System,” EPA News Release, 04/16/2016, https://www.epa.gov/newsreleases/epa-deq-and-city-flint-recommend-flushing-water-speed-recovery-system (May 17, 2016).