The microelectronic devices used in the automotive industry are very important, as is the case of the control unit that activates the airbag of a vehicle when traffic accidents occur. The airbag system can fail to activate when these devices experience corrosion, and injuries to persons inside the vehicles are not minimized as intended. In this study, the corrosion of the control unit electronics of an airbag was related to their assembly in indoor facilities without environmental controls. Atmospheric factors included relative humidity (RH) higher than 75% and temperatures above 30 °C, common in the summer and winter seasons in Mexicali, Baja California, Mexico. In addition, sulfur dioxide (SO2) exceeded the levels of air quality standards (AQS), and nitrogen oxides (NOx) and ozone (O3) were near the upper limits of the AQS during the summer and winter months.
Mexicali, located in the northwest of Mexico, is considered an arid zone and one of the most polluted cities in this country, principally due to air pollutants derived from sulfur. The main sulfur emission source (as hydrogen sulfide [H2S]) is from the geothermal-centric industry supported by the Cerro Prieto volcano, which supplies electricity to the Mexicali city and valley and some cities in the southwest United States. H2S is a corrosive and toxic pollutant. Other emission sources are from vehicular traffic and industries located in Mexicali, which include ~20 automotive plants. The NOx is emitted from the thermo-electrical industry plant and the vehicles, and the O3 is formed by reactions of these pollutants with the air.
Automobile Electronic Systems
The use of electronic devices and equipment in automobiles has increased during the last 30 years because the reduced size of these components facilitates their inclusion in cars that are smaller and more fuel-efficient. These electronic systems must meet very specific operational requirements for proper operation. In Baja California, there is approximately one car for every five inhabitants (~500,000 automobiles). In this area of Mexico, corrosion occurs very rapidly and affects the operation of motor vehicle electronic systems.
Climatic Factors
In arid areas such as Mexicali, moisture content in indoor environments of automotive manufacturing facilities modifies the electrical properties of electronic systems used in automobiles, such as the microdevice that activates the airbag. Variations in air moisture—dry in the summer months of July and August and wet in the winter months of December and January—strongly increases deterioration. Climate is comprised of several parameters, with RH and temperature being the most important factors that can contribute to corrosion damage of electronic components. Some scientists who analyze deterioration of materials in connection with climatic and environmental conditions consider that operational problems with electronic systems in cars are related to drastic seasonal changes in RH and temperature inside industrial plants, as mentioned in the evaluation of the automotive industry in Mexicali1 and expressed in ISO 9223.2
Atmospheric Pollution
Gaseous and solid airborne pollutants, including H2S and SO2, generate aggressive environments in combination with levels of humidity and temperature that are higher than 75% and 30 °C, respectively. This initiates the corrosion phenomena that damages metallic components of electronic equipment in motor vehicles. Arid zones, as in Mexicali, present RH that typically ranges from 30 to 90% in summer with temperatures higher than 40 °C. Winter temperatures are lower than 5 °C and RH ranges from 45 to 85% inside the automotive plants. The efficiency of the electrical behavior of materials used in the electronic devices is a function of the amount of humidity and pollutants present in the indoor assembly environment. The most active gases are H2S and SO2.
Auger Electron Spectroscopy Technique
The Auger electron spectroscopy (AES) technique was used to analyze, at the nanoscale, the corrosion deterioration of the electronic microdevice that controls the motor vehicle’s airbag activation. With this technique, it was possible to detect the particles that reacted with the electrical connections of the microdevice and caused the electrical failures. Modern microelectronic devices and equipment in automobiles have very complex designs, with multiple operations combined with small size. These devices use specialized materials with corrosion-resistant characteristics. The AES technique was used to evaluate the wide range of materials, layers, sizes, and geometries of the corroded microdevices. It is necessary to understand the use of this technique so that parameters such as applied voltage and current can be controlled so layers that are critical to the analysis are not destroyed. The AES technique was also utilized to evaluate new materials, standardize new methods of manufacturing, and improve their corrosion resistance and operability of the electronics.
Experimental Procedure
A mathematical correlation was made using MatLab† software to determine the corrosivity levels in the automotive industry’s indoor facilities.3 This analysis, which was correlated with climate factors (humidity and temperature) and air pollutants (H2S and SO2), determined the deterioration grade and corrosion rate (CR) of the corroded connectors in the microdevice used in the airbag system. These climate factors entered the indoor environment from outdoors through inlets or air conditioning systems. The Auger spectra of the corroded microdevice were analyzed using the 5-keV electron beam and 10 μA of electrical current.4 The AES spectra show the surface analysis of three points evaluated in different zones of a corroded microdevice. The spatial resolution of this technique was ~100 nm with a resolution depth of 1 nm.
Results
Corrosion products between connection points caused failures in electronic systems of the motor vehicles. Specific electronic devices operate at macro- and microscales and are fabricated in dedicated, but not isolated, rooms with particular materials and methods selected to resist corrosion. Nevertheless, the absence of environmental controls allowed intrusion of the corrosive atmospheric factors. In winter, uniform corrosion was promoted by the wetting film formed on the metallic surfaces of some microdevices. In summer, minor tarnishing appeared on components as a result of air pollutants and droplets that adhered to metal surfaces, which resulted in pitting corrosion.
Corrosivity Level Analysis
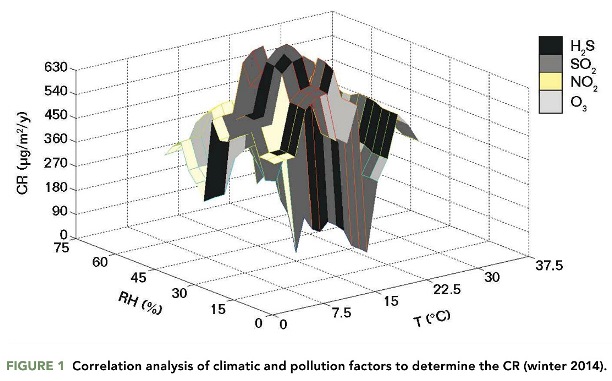
The corrosivity levels in the Mexicali automotive industry indicated the presence of an aggressive environment. The CR calculated from the atmospheric pollution in indoor facilities indicated that the air pollutants caused damage to the metallic surface within the microdevices and prevented effective airbag deployment. The macro- and microelectronic systems of the automobiles corroded rapidly in the high-humidity environment. Figure 1 shows the correlation of ranges of RH and temperature correlated with the concentration levels of SO2, the dominant air pollutant. The RH and temperature in the winter months ranged from 30 to 75% and 20 to 35 °C, and the annual CR ranged from 30 to 100 μg/m2. These were the largest seasonal CRs.
Auger Electron Spectroscopy Examination

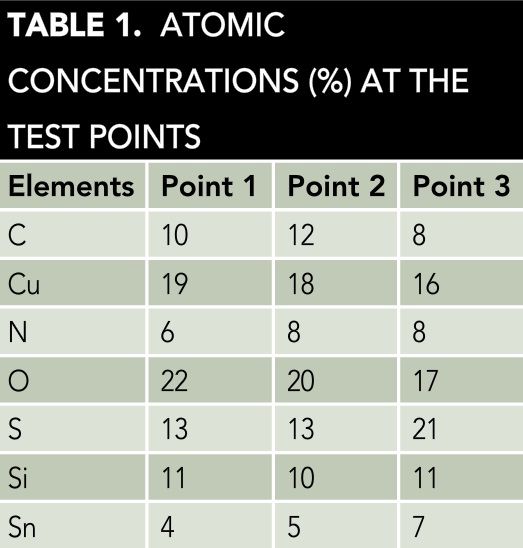
The AES analyses determined the corrosion products formed on the metallic surfaces of the damaged microdevices. Figure 2(a) shows the map of the AES image of the deteriorated zones of the microdevice. Three principal points were selected for evaluation with the AES technique. Figure 2(b) presents the spectra of the AES analysis. The plots in Figure 2(b) were drawn using Sigma† software. The AES map indicates the chemical compounds in the selected points are composed of the main corrosive ions present on the metallic surfaces.4 The principal metals identified on the surfaces are Cu attached to Sn. The atomic concentration (%) of the chemical elements in each spectrum is listed in Table 1.
The concentration percentages in Table 1 represent the chemical elements that comprise the corrosion products, which are primarily derived from the H2S and SO2 pollutants.
Summary
This study was initiated to determine why a system failed to deploy an airbag during testing. Modern advances in electronic components have resulted in the development of much smaller units; however, that also means the much-smaller conductors and connectors are less tolerant of corrosion. Factors contributing to corrosion include the uncontrolled climates that intrude on indoor environments.
As a result, corrosion in microelectronic devices used in the automotive industry has been increasing during the last five years. This corrosion process involves the formation of galvanic electrochemical corrosion cells, which have been found to cause electrical failures during testing. Cu is the most common material used in the electronic control devices because of its good electrical and thermal conductivity; however, the copper microcircuits, electrical connectors, and connections are very susceptible to atmospheric corrosion under the conditions found in Mexicali. The formation of corrosion products on the surfaces of copper connectors is a particular concern because it can result in poor electrical contacts that fail to properly conduct the necessary current/signal to a component.
In most cases, companies are not familiar with the phenomenon (or consequences) of corrosion, until it causes a failure in electronic devices or equipment and interrupts the manufacturing process. This case history is one such example, which helps to demonstrate the value of environmental control for indoor facilities.
†Trade name.
Acknowledgments
The authors thank the companies of the Mexicali automotive industry for the support received for research and the information supplied to the study.
References
1 G. Lopez Badilla, “The Characterization of Corrosion in the Electronics Industry of Mexicali,” Doctoral thesis, Universidad Autónoma de Baja California, 2008.
2 ISO 9223:1992, “Corrosion of metals and alloys—Corrosivity of atmospheres—Classification” (Geneva, Switzerland: ISO, 1992).
3 E.B. Magrab, et. al, An Engineer’s Guide to MatLab, 2nd ed. (Upper Saddle River, NJ: Prentice Hall, 2005).
4 A.E. Clark, C.G. Pantan, L. Hench, “Auger Spectroscopic Analysis of Bioglass Corrosion Films,” J. Am. Ceram. Soc. 59, 1 (2006): pp. 37-39.
Bibliography
1999 ASHRAE Handbook: HVAC Applications. Atlanta, GA: ASHRAE, 1999.
Asami, K. and K. Hashimoto. “AES Study of the Corrosion Behavior of Metallic Surfaces.” Corros. Sci. J. 39, 1 (2007): pp. 95-106.
Briggs, D. and M.P. Seah. Practical Surface Analysis, Auger and X-ray Photoelectron Spectroscopy, 2nd ed., Vol. 1. Hoboken, NJ: Wiley, 1990.
Dillon, P. “MTI & DOE Launch Project Partnerships.” MTI Communications (2000).
“Wear and Erosion: Metal Corrosion,” Annual Book of ASTM Standards. Vol. 03.02. West Conshohocken, PA: ASTM, 2000.