NACE/ASTM G193-12D1 defines a corrosion inhibitor as “a chemical substance or combination of substances that, when present in the proper concentration and forms in the environment, reduces the corrosion rate.” ICRI Concrete Repair Terminology (2017)2 defines a surface-applied corrosion inhibitor (SACI) as “a chemical compound that, when used as a topical application to hardened concrete in the proper concentration and form, prevents or reduces corrosion.” There are four components of a corrosion cell: the anode, the cathode, an electrical path (steel), and an ionic path. Changing any one or more of these components changes the corrosion rate. More than one inhibition/mitigation technique can work at the same time to reduce the rate of corrosion to an acceptable level. For example, cathodic protection combined with a coating changes the anode, cathode, and electrical path. In cured concrete, keeping water out by interrupting the ionic path also helps address many of the durability issues of concrete, including corrosion.
Surface-Applied Corrosion Inhibitors
SACIs are one of the simplest methods to address corrosion issues with reinforced concrete, although there does not appear to be any industry-accepted protocol for evaluation of effectiveness or comparison of materials. In this study, tests were selected from published information from different commercial proprietary products and performed at independent testing laboratories.
There are several general technologies used for SACI. Water-based amino alcohols can be ponded onto the surface and are claimed to also have enough vapor pressure to penetrate as a vapor into the concrete.3 Amino alcohols remain water soluble and are likely to have at least as much vapor evaporating into the outside environment as can slowly penetrate the concrete. Water-based materials can also leave a residue on the surface of the concrete that must be removed before coatings or overlays are applied. This explains the mixed results of water-based SACIs.
Silane-based materials react with the concrete binding to the pores, producing a water-repellant barrier to chlorides and most other deterioration mechanisms. The benefits of silane treatment include being one of the most cost-effective protections for concrete, and that it has a negligible impact on the application of most coatings, overlays, or other materials onto the treated surface. Silane treatments also do not change the appearance of the concrete, merely repelling liquid water from penetrating after reacting while allowing water vapor to evaporate.4 Unfortunately, silane treatment of concrete is generally not permanent and typically requires reapplication every five to 10 years to maintain the water repellency.
Combining silane with a corrosion inhibitor has been very successful in producing a synergistic effect.5-6 For corrosion inhibitors to work, they must reach the steel surface to be protected. While silane remains on the surface, penetrating up to a few inches depending on the treatment, the corrosion inhibitor penetrates the concrete along with the silane treatment by capillary suction and can either travel as a vapor phase into the concrete or remain latent until the water repellency decreases. Latent water-soluble corrosion inhibitors that do not react with the silanes are dissolved into the silane mixture and move into the concrete along with the silane treatment. There they remain until the silane is no longer providing water repellency to the concrete. Once water can penetrate the concrete, in most cases when a crack appears, the water-soluble corrosion inhibitors move along with the first few drops of liquid water to reach the steel and then provide latent corrosion protection.
Experimental Procedures
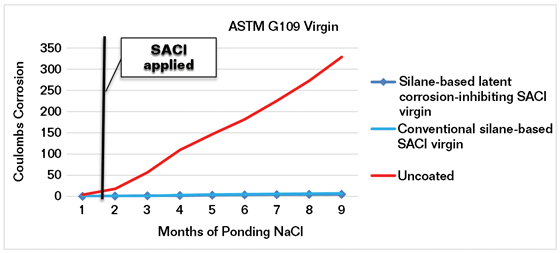
ASTM G1097 is a test method for “determining the effects of chemical admixtures on the corrosion of metals in concrete” (Figure 1). A modification of ASTM G109 testing was performed where the concrete specimens were made without admixtures and were abrasive blasted to ICRI CSP 5-68 after curing and then coated with different silane-based SACIs, one with a latent corrosion inhibitor and the other a conventional SACI. Ponding with G109 3% sodium chloride (NaCl) solution was initiated one week after SACI treatment. In this testing, both SACI materials work well, as shown by the total coulombs of corrosion current between the upper and lower bars (Figure 2).
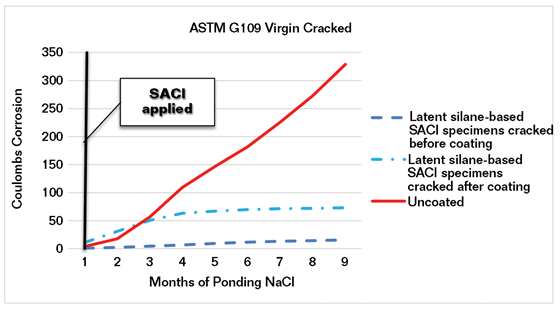
In another series of tests, G109 specimens were cracked before the SACI was applied and additional specimens were coated with the latent inhibitor SACI before cracking. Ponding with 3% NaCl for two weeks, followed by drying at 50% relative humidity and measuring the accumulated corrosion current, is shown in Figure 3.
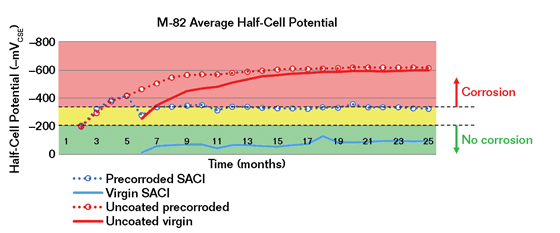
Another test of corrosion mitigation is described in U.S. Bureau of Reclamation publication M0820000.714.9 Slabs were divided into virgin and precorroded specimens that were cast from the same concrete truck, identically cured, and abrasive blasted. Precorroded slabs were then cyclically ponded with 3% NaCl followed by drying at two-week intervals for four months. The latent SACI was applied to half of the virgin slabs and half of the precorroded slabs, with the remaining slabs considered control specimens when ponding continued. The virgin, precorroded, and control specimens were tested using ASTM C87610 using a copper/copper sulfate (Cu/CuSO4) electrode (CSE) and corrosion current measured between the upper and lower layers of reinforcement. Both virgin and precorroded uncoated control specimens showed a continued increase in corrosion activity, while both series of virgin and precorroded specimens treated with the latent corrosion-inhibiting SACI showed significantly reduced corrosion (Figure 4).
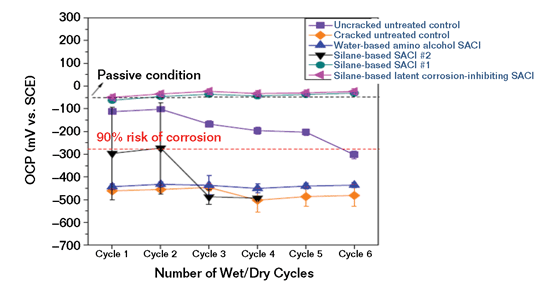
In another test series, 18 ASTM G109-type specimens were then cast and cured. Six G109 blocks were selected for control specimens. Groups of three other blocks had different commercial SACI treatments applied per the SACI manufacturer’s recommendations, including a water-based amino alcohol, a conventional silane SACI, and the latent inhibitor silane-based SACI. Three of the untreated blocks along with each of the treated blocks were cracked perpendicular to the top reinforcing bar before ponding with 3% NaCl solution. After each cycle of drying and ponding, corrosion measurements using ASTM C876 half-cell testing were taken prior to the dry cycle. ASTM C876 provides guidance in an appendix regarding interpretation of the readings (Figure 5).
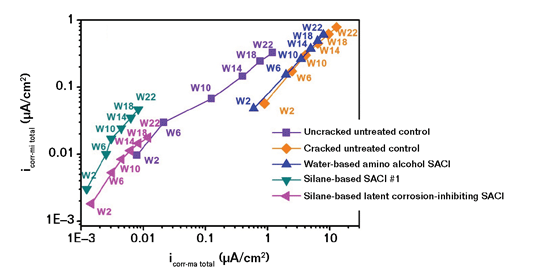
Another test is electrochemical impedance spectroscopy (EIS).11 The results in Figure 6 show microcell corrosion current density (CD) based on concrete resistance and polarization resistance in EIS measurements vs. total macrocell corrosion CD based on G109 measurement. In both the open circuit potential testing on cracked specimens in Figure 5 and the EIS testing in Figure 6, a significant difference is shown between the SACI products.
Summary and Conclusions
Corrosion of reinforcing steel is the leading cause of deterioration of concrete structures around the world. Guidance exists for design and construction practices to produce durable structures; however, corrosion-induced deterioration continues from failure to follow good trade practices. Concrete requires maintenance, especially to correct issues from cracking.
Use of SACIs is a maintenance technique that can be applied proactively to existing structures as well as reactively to address incipient corrosion. Silane-based materials reduce chloride ingress and allow concrete drying to increase resistivity, both reducing corrosion. Incorporating latent corrosion inhibitors into silane-based surface treatments combines the benefits resulting from silane treatments and the corrosion inhibiting effects in concrete that cracks even after treatment. Specific methods suitable for the silane-based latent inhibitor SACI have been described along with comparative testing of several other commercial SACI materials.
Future Work
The conclusions based on the corrosion testing are tentative. Much longer-term testing, including breaking open the specimens (autopsy) when cracking develops, will be required to demonstrate the longterm effectiveness of these treatments.
References
1 NACE/ASTM G193−12d, “Standard Terminology and Acronyms Relating to Corrosion” (Houston, TX: NACE International, 2013).
2 “Concrete Repair Terminology” (St. Paul, MN: ICRI, 2017).
3 F. Wombacher, U. Maeder, B. Marazzani, “Aminoalcohol Based Mixed Corrosion Inhibitors,” Cement and Concrete Composites 26, 3 (2004): pp. 209-216.
4 P.D. Carter, “Evaluation of Dampproofing Performance and Effective Penetration Depth of Silane Sealers in Concrete,” Special Publication 151 (Farmington Hills, MI: ACI International), pp. 95-118.
5 N.S. Berke, et al., “Organofunctional Silane Corrosion Inhibitor Surface Treatment of Concrete to Mitigate Corrosion due to Chlorides or Carbonation,” CORROSION 2017, paper no. 9130 (Houston, TX: NACE, 2017).
6 A.I. Karayan, et al., “Evaluation of Different Surface Applied Corrosion Inhibitors Systems on Reinforced Concrete Structures Based on Electrochemical Techniques and Standard Testing,” Dept. of Defense Allied Nations Conference, paper no. 2017-0000 (2017).
7 ASTM G109 (latest edition), “Standard Test Method for Determining Effects of Chemical Admixtures on Corrosion of Embedded Steel Reinforcement in Concrete Exposed to Chloride Environments” (West Conshohocken, PA: ASTM International).
8 ICRI Guideline 310.2R (latest edition), “Selecting and Specifying Concrete Surface Preparation for Sealers Coatings and Polymer Overlays” (St. Paul, MN: ICRI).
9 M-82 (M0820001.714), “Standard Protocol to Evaluate the Performance of Corrosion Mitigation Technologies in Concrete Repairs” (Denver, CO: U.S. Dept. of the Interior, Bureau of Reclamation, 2014).
10 ASTM C876 (latest edition), “Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete” (West Conshohocken, PA: ASTM).
11 D.V. Ribeiro, C.A.C. Souza, J.C.C. Abrantes, “Use of Electrochemical Impedance Spectroscopy (EIS) to Monitoring the Corrosion of Reinforced Concrete,” Revista IBRACON de Estruturas e Materiais 8, 4 (2015): pp. 529546.
This article is based on CORROSION 2018 paper no. 10515, presented in Phoenix, Arizona, USA.