Current standard test methodologies used in determining the vapor inhibition ability of volatile corrosion inhibitors (VCIs) provide only qualitative results. They are also unsuitable for evaluating the ability of the different VCIs in providing corrosion protection to pre-corroded ferrous metals.
In this study, a rapid and quantitative test method was developed using coupled multielectrode array sensor technology. The results obtained showed the ability of this test method to provide consistent results and differentiate quantitatively between the anti-corrosive effects of the different VCI materials on clean and pre-corroded ferrous metals.
Metallic corrosion is a natural inevitable phenomenon defined commonly as the deterioration of metals due to reactions with their environments. The global cost of corrosion estimated by NACE International in 2013 was found to be $2.5 trillion (USD), which is approximately 3.4% of the global gross domestic product (GDP).
The two-year global study released at the CORROSION 2016 conference in Vancouver, British Columbia, Canada, assessed the economics of corrosion and the role of corrosion management in establishing best practices for the different industrial sectors. It found that implementing corrosion prevention best practices could result in global annual savings of 15 to 35% of the cost of damage, which is equivalent to $375 to 875 billion (USD).
These estimations excluded the cost of individual safety and environmental consequences from corrosion. Corrosion mitigation has been extensively researched. The methods of corrosion prevention include, but are not limited to, selection of the right material of construction, coatings, corrosion inhibitors, and cathodic protection (CP).1-2
One class of corrosion inhibitors used in corrosion protection applications is volatile corrosion inhibitors (VCIs). They are a class of organic chemicals that possess appreciable saturated vapor pressure under atmospheric conditions. They protect ferrous and non-ferrous metals from corrosion by vapor transport of inhibitive molecules from a VCI material in an enclosed environment, such as packaging, shipping, and storage of metal parts.
This vaporization is followed by diffusion through the electrolyte layer that presents on the surfaces of metals and formation of a thin protective film, which isolates the metal surface from the corrosive environment by enhancing the hydrophobic properties of the metallic surfaces.
The transport of the VCI to the metallic surface can take place in two ways: 1) the inhibitor dissociates and saturates the air in contact with the metal with the protective groups, or 2) the inhibitive molecules volatilize without dissociation and dissociate only when they reach the metallic surface.
The main advantage of VCIs compared to contact corrosion inhibitors resides in the ability of the inhibitor molecules to form the protective film on the metal surface, including hard-to-reach areas, without being in direct contact with the surface.3-4
As in the case with conventional corrosion inhibitors, a prerequisite for qualifying a VCI material for field application is to evaluate its vapor-inhibiting ability (VIA). Several testing methods have been proposed for rapid evaluation of VIA of various forms of VCIs for corrosion protection of ferrous metal surfaces, such as NACE Standard TM0208,5 MIL STD 3010-4031,6 German TL 8135 (papers and films),7 and Japanese JIZ S 1535 (papers).8
These tests are relatively simple. They involve the use of a ferrous metal specimen suspended in a closed container getting exposed to a sequence of three main environmental conditions: 1) conditioning accompanied with or followed by introduction of a high-humidity environment, 2) aggravation of the humidity environment by introducing heating and cooling cycles, and 3) visual evaluation of the level of corrosion formed on the specimen and grading it against a typical rating diagram.
A general review paper provides perspective on the need for the more recent NACE Standard TM0208 and issues with attempts within NACE to create a similarly simple and quick VIA test for prevention of corrosion for various types of non-ferrous metal specimens.9
In spite of the fact that these testing methodologies share many similarities, their results can also vary significantly, leading to different conclusions about the efficiency of the inhibitor. They also have intrinsic difficulty in producing consistent results. J. Brekan et al. discussed some pitfalls and surprising results in several routine VIA tests.10
It was found that minor variations in the quality of sandpapers and type of solvents used by the test operator to prepare the specimens, as well as the conditioning temperature, have a major effect on the results and can lead to false negative or positive conclusions. Furthermore, such qualitative tests are useful to assess the efficiency of VCIs on freshly prepared (clean) specimens and for corrosion. They cannot be applied to evaluate the performance of a VCI against localized corrosion and on rusted metal surfaces.10
Several studies used electrical resistance probes as a means to quantitatively evaluate the performance of VCI materials in specific environments such as storage application in atmospheric conditions and simulated wet gas pipeline “top of line corrosion” in laboratory experimentation.11-12
However, this technique has two fundamental limitations to be used in VIA tests, which are: 1) the inability to reuse the probe for multiple tests, which makes it cost prohibitive; and 2) the relatively slow response rate in low corrosive environments. Another candidate technology that can be used for quantitative evaluation of VCI performance is coupled multielectrode array sensors (CMAS). This technology has been utilized effectively as a quantitative tool for online and real-time monitoring of the rate of corrosion in laboratory experimentations as well as industrial fields.
CMAS technology was used extensively in testing non-uniform (localized) corrosion of metals and alloys in various environments such as carbon steel (CS), stainless steel (SS), copper, and aluminum in simulated seawater, CS in crude oil/water mixtures, deposits of salts in air, sulfate reducing bacteria solutions, concrete, and hydrogen sulfide (H2S) systems. Furthermore, they were used to evaluate the effectiveness of CP and degradation of coatings.
In addition to the localized corrosion, CMAS probes were used to estimate uniform/general corrosion rates of CS and aluminum in 2 wt% hydrochloric acid (HCl) solution, as well as CS, brass, and SS in low-conductivity waters. In addition, CMAS technology was used to evaluate the effect of contact corrosion inhibitors on CS in a simulated cooling water environment.13-15 The use of CMAS probes for quantitative evaluation of the presence of the vapor inhibiting ability of VCIs will be evaluated.
Experimental Procedure
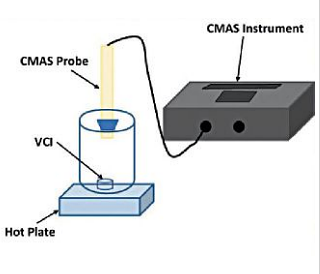 Before each experiment, the CMAS probe was polished with sandpaper, rinsed with deionized water and methanol, dried using lint-free wipes, and fixed through the hole on the lid, as shown in Figure 1.
Before each experiment, the CMAS probe was polished with sandpaper, rinsed with deionized water and methanol, dried using lint-free wipes, and fixed through the hole on the lid, as shown in Figure 1.
To test the efficiency of the VCIs on a clean probe, the jar was left empty or with the addition of 1.8 mL liquid VCI or 0.9 g VCI powder for a conditioning period of two days at room temperature. Then, 18 mL of 3 wt% glycerol solution were added to the jar (approximately 100% relative humidity). After 2 h, the jar was placed on a hot plate to achieve 35 ± 5 °C for two days.
To study the effect of VCIs on a pre-rusted probe, the glycerol solution was added from the beginning of the experiment and left for 2 h. Then, the jar was placed on the hot plate at 45 ± 5 °C air temperature inside the jar for two days, followed by the addition of the VCIs for another two days. Each experiment was conducted in duplicates (using two CS probes). The VIA efficiencies were calculated according to Equation (1):

where (Avg CR)Control and (Avg CR)VCI were the average non-uniform corrosion rates from the CMAS probes and each of them was the average value over the two-day heating period.
Results
Clean and Corrosion-Free Probes
Figure 2 and Table 1 show the average CR and efficiency of two runs for the control as well as every VCI material. VCI-C (powder form) showed the highest efficiency of 97.9%, followed by VCI-B (water-based) at 90.1%, and VCI-A (water-based) at 68.1%.
Water-based VCI materials showed a transient increase in the corrosion rate after heating, which can be attributed to partial protection for some of the electrodes due to the low vapor pressure of the VCI material in these liquid inhibitors, where the transport of corrosion-inhibiting molecules are assisted by the solvent vapor.
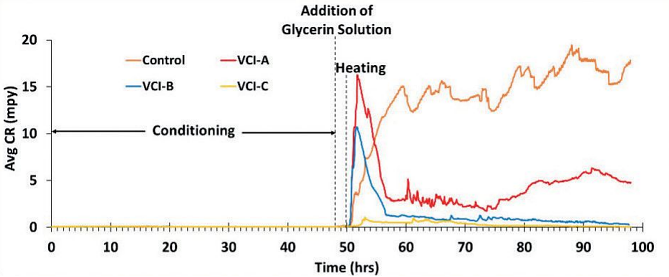
In addition, the probes were able to differentiate between the efficiency of the two water-based inhibitors, which can be attributed to VCI-A having lower vapor pressure than VCI-B. The appearance of the electrodes of the probes immediately after the test complies with the numerical value of the efficiency calculated.

Pre-Corroded Probes
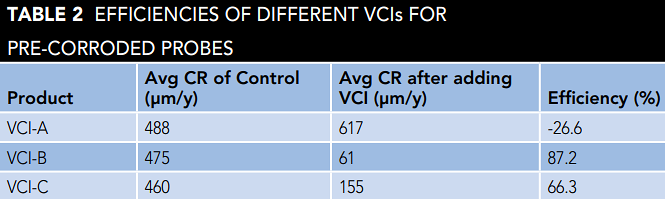
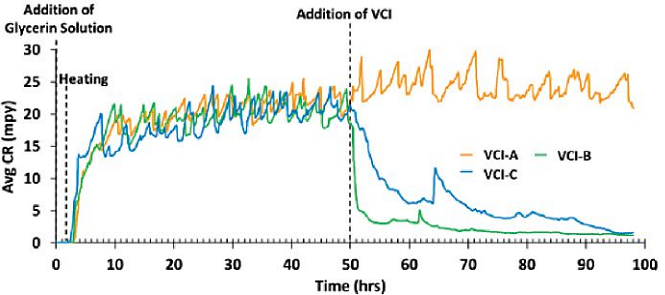
The ability of VCI- A, B, and C to reduce the active corrosion rate on a pre-corroded surface was evaluated and plotted in Figure 3. The results show that VCI-B and C were able to reduce the active corrosion rate and provide protection for pre-corroded steel.
VCI-A, on the other hand, could not reduce the corrosion rate and provide any protection in the case of pre-corroded surface, as it did with clean and rust-free probes (Table 2). In fact, the results show that VCI-A resulted in a slight increase in the corrosion rate, which can be attributed to the increase of conductivity of the electrolyte layer on the probe surface by the VCI molecules that reached and were not able to diffuse through the existing layer and reduce the corrosion rate.
The results also show VCI-B performed faster in reducing the active corrosion rate compared to VCIC, which eventually converged toward the end of the experiment. This, in turn, may show that VCI-B has a faster diffusion rate in rust layers than VCI-C.
Conclusions
With the limitations of the current standard qualitative tests used to determine the presence of the vapor inhibiting ability of VCI materials represented by the inability of these tests to differentiate quantitatively between the performance of the different inhibitors and their inapplicability to evaluate the anti-corrosive effect of VCI materials on pre-corroded steel, there is a need in the industry to develop a quantitative VIA test method to investigate the efficiency and behavior of the different VCI materials.
In this article, CMAS technology was utilized successfully and was able to achieve the following:
• Provide consistent and repeatable results for the control and all VCI materials in consideration
• Differentiate quantitatively between the anti-corrosive effects of the different VCI materials
• Indicate performance differences between VCI in powder vs. liquid forms in a VIA testing environment
• Determine VCI materials that can reduce the active corrosion rate and provide protection to pre corroded surfaces.
Acknowledgment
The authors wish to acknowledge the assistance of Dr. Mohamad Nagi at the American University of Dubai for the test facilities.
Sources: Cortec Middle East, www.cortec-me.com; Corr Instruments, LLC; www.corrinstruments.com.
References and About the Authors