Researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory (Lamont, Illinois, USA) are working on the development of a new oil-resistant filter technology to prolong the life of equipment in industries such as oil and gas.
The new invention1 consists of a type of coating that produces thin films of oil-repelling molecules on the surface of filter membranes. According to the researchers, the metal oxide molecules grab onto any loose water atoms while resisting oil.
Science Behind the Technology
The research team identifies the twin scientific properties of their surface coating system as hydrophilicity and oleophobicity.
“One of the best ways to clean oily water is with membranes,” says Seth Darling, director of the Institute for Molecular Engineering at Argonne. “The problem is that the oil sticks on the membrane and clogs the holes until the membrane stops working.”
“Today, if people have an oil-fouled membrane, either they replace it or they try to clean it with harsh chemicals to wash away the oil,” he adds. Many of these chemicals can be corrosive.
In their work, the scientists utilized atomic layer deposition, which uses chemical vapors to deposit a very thin coating of the metal oxide on the filter membrane surfaces. From there, the researchers experimented using different metal oxides on off-the-shelf commercial polymer membranes to find which ones worked the best. While atomic layer deposition itself is not a new technique, Darling says it has never been previously used in this manner to modify membranes.
“It’s kind of cutting edge,” Darling says. “The coating is just a few nanometers in thickness. If the coating were thicker than this, it would close off the tiny pores. What you want is a minimal change of the pore structure, but you want to change the chemistry of the substance lining those pores.”
In prior studies, scientists tried to create this layer by attaching nanoparticles to a membrane by flowing them through it or growing them on it. But particles tend to get ripped off as water flows through those systems, the researchers explain.
By contrast, they say atomic layer deposition is different because the metal oxide film forms strong chemical bonds with the polymer to which it is adhered. In this process, the membrane is exposed to a sequence of vapors that stick molecules together, forming covalent bonds with the polymer.
“Some polymers bind more easily than others, and some repel oil while others do not,” Darling says of his group’s process. “At this point, we have a pretty good sense of which ones work and why.”
Tin oxide (SnO2) and titanium oxide (TiO2) formed the tightest bonds with water molecules, capturing them and layering them across the surface. “When oil contacts the membrane, it will stay separate because it flows over the water layer,” says Hao-Cheng Yang, a postdoctoral researcher working on the project.
Potential Fracking Use
Fouled membranes can be a costly hassle for the oil and gas industry, according to the researchers. For instance, when oil companies replace clogged filters during the hydraulic fracturing process—or fracking—they must shut down equipment to make the change.
Oil-resistant membranes like this could significantly reduce the need for both filter replacement and the downtime it creates, says John Harvey, Argonne’s business development executive handling the technology.
“Just from my knowledge of the oil and gas sector, if we could make a membrane that performs to even a fraction of what we’ve seen in lab testing, it will be a phenomenal improvement over what’s available now,” Harvey explains.
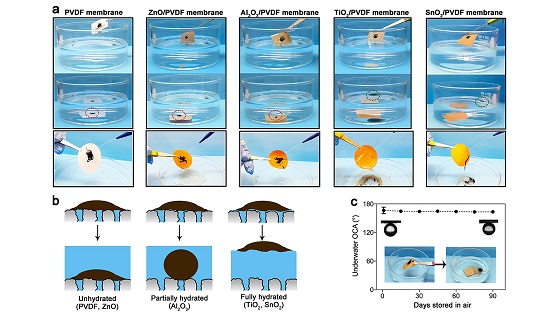
Another problem in the oil and gas industry involves the water used in fracking operations, which often returns from the ground with oil, salt, and other contaminants present. Contaminated water cannot be returned to the ground if it poses a threat to aquifers, so the industry often must find another way to dispose of it.
The membranes used now can remove the other contaminants, but they become fouled by oil, according to the research team. On the other hand, the atomic layer deposition process keeps the membranes from clogging to better filter the water passing through them.
“With this technique, they can keep using that water,” Harvey says. “This could be a direct replacement for filter units they’re using today.”
Other Plausible Applications
Aside from fracking, the researchers believe the method potentially could help with oil spill cleanup efforts. In an oil spill response, diesel fuel is used as a cleaning agent on pipes and containers, which leaves a waste of diesel mixed with oil and dirt. But pipe and container surfaces treated with the oxides could just be rinsed clean, Darling says.
Darling, who also invented the Oleo Sponge to soak oil out of seawater, believes the two technologies could potentially be used in concert for future cleanups—and for a host of other applications.
“One thing I learned from the Oleo Sponge is that you can’t envision all the possible applications at the beginning,” Darling says. “We anticipated interest from oil companies, but we’ve also heard from the cosmetics industry and from sporting goods manufacturers. So I suspect once this gets out, people will also come up with applications we never imagined.”
In addition to Argonne, the scientific team included representatives from the University of Chicago (Chicago, Illinois, USA). Much of the work was performed at the Center for Nanoscale Materials, a DOE Office of Science User Facility, located at Argonne. The research was funded through Argonne’s Laboratory Directed Research and Development Program.
Source: Argonne National Laboratory, www.anl.gov.
Reference
1 “Argonne Scientists Create New Oil-Resistant Filter Technology,” Argonne News & Announcements, Oct. 25, 2018, https://www.anl.gov/article/argonne-scientists-create-new-oilresistant-filter-technology (Dec. 18, 2018).