Vapor phase corrosion inhibitors (VCIs) are a corrosion inhibitor technology that is comprised of very small particles that are attracted to a metal substrate. Once the particles attach to the metal substrate through adsorption, they prevent a corrosion cell from forming. They come in various formulations that are dependent on the type of system they will be used in; for example, films, oils, coatings, cleaners, etc.
There are also a variety of formulations that provide protection in ferrous, nonferrous, or multimetal applications. Other variables include the amount of vapor phase compared to contact phase inhibitors.1 VCIs are widely used throughout a broad range of industries and applications ranging from automotive to processing to preservation and have saved billions of dollars of corrosion expenses.
VCIs as Alternative Corrosion Inhibitor Technologies
The use of VCIs as alternative corrosion inhibitor technologies in coatings is not a new concept. In the last few years, however, with the growing environmental pressures to reduce the use of traditional inhibitors containing heavy metals, they have gained in popularity.2
VCIs as a category are very broad and can be made up of thousands of combinations of raw materials that can have varying rates of effectiveness. Commonly used terms, such as amine carboxylates, cover a broad range of potential formulations. Depending on the formulation, they can vary in their functionality as far as contact vs. vapor phase inhibition. When choosing the right VCI package to formulate into a coating, it is critical to find not only the package that is compatible with the coatings carrier (solvent or water) but also the resin system.
Choosing the wrong inhibitor package can lead to a variety of issues in the coating itself, which include gelling, phase separation, and flocculation. Once these issues have been eliminated, the next stage is testing to determine at which level there is an improvement in the corrosion performance, which is typically done using the salt fog test standard (ASTM B1173).
Since VCI particles have a polar attraction to the metal substrate, this allows them to work in the coating without negatively impacting other components of the coating such as defoamers, wetting agents, leveling agents, etc. VCIs are typically added to the formulation in very small amounts by weight of the overall formula. The typical range is from 0.5% to 3%.
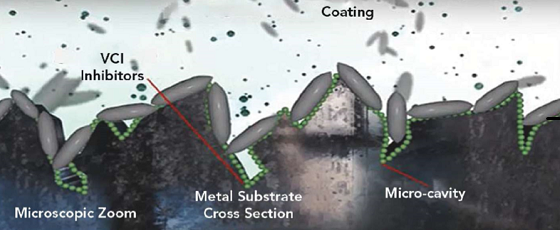
The particle size of the VCIs is very small in comparison to the traditionally used inhibitors (Figure 1). This allows the VCIs to migrate into the smaller voids more effectively.
Once the VCIs have adsorbed onto the surface of the metal, they provide an effective barrier that is hydrophobic and prevents moisture from getting through to the metal surface. Consequently, this prevents the formation of a corrosion cell and renders the moisture ineffective.4
Experimental Procedure
These studies examine the effectiveness of various types of corrosion inhibitors in single-component, waterborne acrylic coatings, based on salt fog results (ASTM B117). ASTM B117 tests products in a 5% sodium chloride (NaCl) salt fog chamber with continuous exposure as per the ASTM standard.
Each coating was applied on cold-rolled steel (CRS) panels (SAE 1010), using a 0.40 drawdown bar. Dry film thicknesses (DFTs) yielded were 0.9 to 1.2 mils (23 to 30 µm). Each coating/inhibitor combination was applied in triplicate. Coated panels were air dried in lab conditions at an ambient temperature of 70 °F (20 °C) and 50% relative humidity for seven days before being placed into the B117 chamber.
A matrix (Table 1) was designed to track the various coating/inhibitor combinations as follows:
- Additive variables:
- Eight different types of “traditional” inhibitors containing zinc phosphates, calcium phosphates, strontium phosphates, etc.
- Products are typically added at a wt% (5%) of the total coating formula.
- Four different types of VCIs containing proprietary blends of amine carboxylates.
- Products are typically added at a wt% (0.5 to 3%) of the total coating formula. For this experiment, they were added at 3%.
- Coatings contained:
- 32 combinations of traditional inhibitors and VCIs.
- Products were added at a reduced wt% (3%) of the total coating formula plus the VCIs at 3%.
- Two combinations with VCIs
- Products were added to a wt% of 0.5 to 2.0% (Figure 2).
Results
The results shown in Table 2 were based on a visual inspection and rating. From the testing that was done, it is clear that VCIs are a viable solution for use as corrosion inhibitors in coatings. Figure 2 shows that VCIs by themselves have the ability to provide excellent corrosion protection. As evidenced, salt spray performance in many cases was matched by reducing the percentage of traditional inhibitor used (recommended dosage of 5% by total formula weight to 3% by total formula weight) and adding the VCI (at 3% by total formula weight). This is illustrated in Table 2 with the positive performing synergies highlighted. These synergies allow for reduced usage of inhibitors that may have to meet stricter environmental limits while possibly providing cost savings as well.
Combinations of B2 with various traditional inhibitors seemed to consistently provide comparable results, while the use of the VCI only provided the best results in this system.
Conclusions
Customers are becoming more and more demanding and are expecting their coatings to last longer. With the ongoing performance and environmental challenges in the coatings industry, there continues to be a need for new technologies that can provide better performance.
Stricter regulations limiting the use of certain products continues to make this more difficult as formulators are having to find alternatives to the products that have been used for many years. This article shows, through research, that the use of VCIs can match or improve the corrosion resistance of coatings either used by themselves or in combination with existing inhibitor technologies, thus reducing the environmental concerns without sacrificing performance.
Acknowledgements
This material is based upon work and research done by Rick Shannon & Alex Hart, Cortec Corporation.
References
1. Y.I. Kuznetsov, et al., “Inhibiting Action and Absorption of Beta-Aminoketones on Metals,” Zasshchita Metallov 32, 5 (1996): pp. 528-53.
2. S. Gangopadhyay, P. Mahanwar, “Recent Developments in the Volatile Corrosion Inhibitor (VCI) Coatings for Metal: A Review” (Washington, DC: American Coatings Association, 2018).
3. ASTM B117-16, “Standard Practice for Operating Salt Spray (Fog) Apparatus” (West Conshohocken, PA: ASTM International, 2010).
4. B.A. Miksic, “Use of Vapor Phase Inhibitors for Corrosion Protection of Metal Products,” CORROSION/83, paper no. 308 (Houston, TX: NACE International, 1983).
This article is based on CORROSION 2019 paper no. 13079, presented in Nashville, Tennessee, USA.