Corrosion is the predominant concern in modern marine engineering, which seriously shortens the service life of offshore structures and easily leads to substantial economic losses.1 Arc-sprayed Al has been extensively used as a protective coating on offshore platforms due to its high costeffectiveness, light weight, and remarkable anticorrosive performance, which is expected to achieve a service life of 30 years in a marine environment.1 Nevertheless, the thermal-sprayed Al coating with typical lamellar microstructure contains many inherent imperfections, such as porosity, microcracks, inter-splat boundaries, oxides, and unmelted particles,2 which reduce the operational reliability of Al coatings in harsh environments.
Laser remelting (LR) as a nonadditive process can effectively reduce or even eliminate the previously mentioned drawbacks. It has several advantages of heat input, economy, and operational flexibility compared with other post-technologies.2 In addition, LR with an extremely fast cooling rate (105 to 108 K/s) meets the required critical cooling rate for forming the amorphous structure, which has been extensively adopted to fabricate the amorphous coatings with dense structure and excellent properties.3-5
In this study, arc-sprayed Al-Ti-Ni coating was processed using a LR at the power of 600, 800, and 1,000 W. The aim was to investigate the effects of power on the salt spray corrosion performances of the obtained coatings.
Experimental Procedures
Sample Preparations
European standard S355 steel was used as the substrate. The chemical composition (wt%) was C 0.17, Si 0.55, Mn 0.94, P 0.035, Cr 0.065, S 0.035, Ni 0.065, Mo 0.30, Zr 0.15, and the balance Fe. The arc spraying test was performed using a DH-600A† type arc spraying machine, using the following parameters: spraying voltage of 30 V, spraying current of 160 A, spraying pressure of 0.6 MPa, spray angle of 80 degrees, wire feeding rate of 2.5 m/min,1 and overlap of 35%. Then, the LR test was performed using a ZKSX† 2,000 W type fiber-coupled laser spraying system. The process parameters were as follows: respective power of 600, 800, and 1,000 W; scanning speed of 8 mm/s; spot diameter of 4 mm; argon gas speed of 15 L/min; and overlap of 50%.
The salt spray corrosion test was performed on a YQW/Q-250† salt spray corrosion chamber, with the following test parameters: spray medium of 3.5% sodium chloride (NaCl) solution, pH range of 6.5 to 7.2, temperature of 30 ± 2 °C, and time of 720 h. The corroded morphologies of Al-TiNi coatings were analyzed using scanning electron microscopy (SEM). The surface morphologies of the obtained coatings were observed using a JEOL JSM-6360LA† type scanning electron microscope.
Analysis and Discussion
Morphology and Energy Dispersive Spectroscopy (EDS) Analysis of Ti-Ni Powder
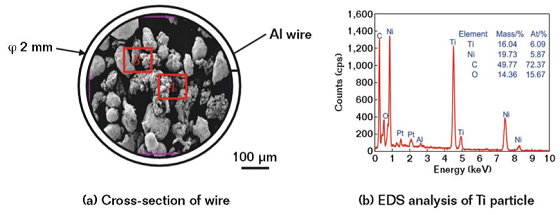
The cross-section morphology of the aluminum-sheathed wire is shown in Figure 1(a). The powder was composed of the Ti particle with the irregular shape and the Ni particle with the near-spherical shape. The chemical compositions of Ti-Ni powder are shown in Figure 1(b). The Ti-Ni powder was mainly composed of Ti and Ni elements, while the C and O were impurities adsorbed in the air.
Morphologies of Coating Surfaces
Figure 2(a) shows the morphology of laser remelted Al-Ti-Ni coating at the power of 600 W. The abundant porosity, unmelted particles, and irregular stacks on the coating surface indicated that the coating was not remelted completely and the defects of arc-sprayed coating were retained.
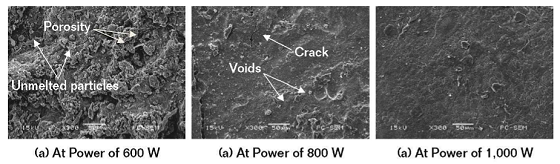
Figure 2(b) shows the morphology of laser remelted Al-Ti-Ni coating at the power of 800 W. The coating surface was flatter at 600 W, with a few voids due to the formation of a gas pocket, and the notable microcracks originated from the thermal stress of solidification.
Figure 2(c) shows the morphology of laser remelted Al-Ti-Ni coating at the power of 1,000 W. The were no visible pores and cracks on the coating, indicating that the arc-sprayed coating was remelted completely to eliminate the porosity and crack entirely.
X-ray Diffraction Analysis
Figure 3 shows the x-ray diffraction (XRD) analysis patterns of laser remelted Al-Ti-Ni coatings at different powers. There were high and narrow crystal phases at 38 to 48 degrees and the intensity of aluminum oxide (Al2O3) peak increased while the intensity of intermetallic compounds (Al3Ti, Al3Ni) disappeared with the increase of power, indicating that the Al2O3 had the preferential formation tendency under the laser action. The broad and low diffraction peaks shown as inserted pictures indicated the presence of amorphous structure.
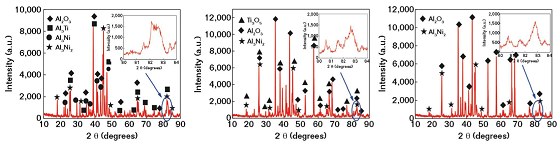
It was reported that the cooling rate during the laser remelting process reached 103 to 105 K/s, which was advantageous to inhibit the formation and growth of grains. Moreover, the differences of atomic sizes between Al, Ti, and Ni were >12% with the almost negative heat of mixture, which met the empirical rules for high glass-forming ability proposed by Inoue.6 It was also worth noting that it was impossible to prepare pure amorphous coatings without a crystalline phase due to the inevitable thermal effect.7 Therefore, the three kinds of coatings by LR were the mixed structure of crystal and amorphous phases.
Corrosion Behaviors
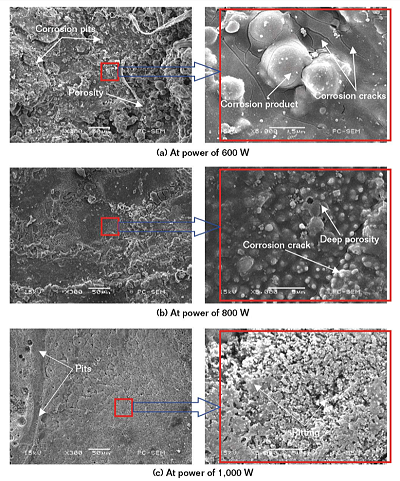 Figure 4(a) shows the corroded morphology of laser remelted Al-Ti-Ni coating at the power of 600 W. There were obvious corrosion pits, porosity, and corrosion products, revealing the severe localized corrosion and irregular corrosion by-products. The abundant irregularities and defects in Figure 2(a) provided effective permeation paths for the electrolyte to reach the substrate, leading to galvanic corrosion between the coating and the substrate,8 which accelerated the corrosion process and led to the massive globular corrosion products preferentially forming at the defect sites. The accumulation of corrosion products constituted a barrier to electrolyte penetration and retarded further corrosion in the early corrosion stages; while the accumulation of corrosion products with the high degree of blocking created such a high pressure that the coating failed with increased corrosion time,9 which generated many cracks around corrosion products.
Figure 4(a) shows the corroded morphology of laser remelted Al-Ti-Ni coating at the power of 600 W. There were obvious corrosion pits, porosity, and corrosion products, revealing the severe localized corrosion and irregular corrosion by-products. The abundant irregularities and defects in Figure 2(a) provided effective permeation paths for the electrolyte to reach the substrate, leading to galvanic corrosion between the coating and the substrate,8 which accelerated the corrosion process and led to the massive globular corrosion products preferentially forming at the defect sites. The accumulation of corrosion products constituted a barrier to electrolyte penetration and retarded further corrosion in the early corrosion stages; while the accumulation of corrosion products with the high degree of blocking created such a high pressure that the coating failed with increased corrosion time,9 which generated many cracks around corrosion products.
The laser remelted Al-Ti-Ni coating at the power of 800 W also suffered from severe corrosion attack, which revealed deep pitting porosity and cracks, as shown in Figure 4(b). The electrolyte assembled on the microcrack and voids in Figure 2(b) formed the pitting nuclei. With continuing corrosion reaction time, the pits began to grow and gradually formed pitting pores. Eventually, the porosity was further expanded and extended along the depth direction.10
The corroded morphology of laser remelted Al-Ti-Ni coating at the power of 1,000 W was relatively flat with the smallsized corrosion pits and pitting, suggesting the lowest corrosion degree among three kinds of coatings, as shown in Figure 4(c). It was also observed that there were only a few corrosion products on the coating surface, indicating that the permeation of corrosive medium was effectively inhibited. The better corrosion resistance was because the defects were effectively eliminated in Figure 2(c), which greatly retarded the infiltration of electrolyte into the coating, and eventually formed the uniform corrosion and pitting corrosion during the corrosion reaction.
As a result, the salt spray corrosion performances of laser remelted Al-Ti-Ni coatings at the power of 600, 800, and 1,000 W were considerably affected by the microdefects. The laser remelted Al-Ti-Ni coatings at the power of 600 and 800 W presented the poor corrosion resistance due to their abundant porosity and cracks; while that at the power of 1,000 W displayed the highest corrosion resistance due to its densification.
Conclusions
1. The laser remelted Al-Ti-Ni coatings are composed of crystal and amorphous phases; among them, the forming tendency of Al2O3 phase increases with increase of power.
2. The salt spray corrosion performances of laser remelted Al-Ti-Ni coatings at the power of 600 and 800 W are mainly controlled by the electrolyte penetrating the surface defects, while that fabricated at the power of 1,000 W is uniform corrosion and pitting corrosion. The compact microstructure is the main factor of improving corrosion resistance.
Acknowledgements
Financial support for this research by the Key Research and Development Project of Jiangsu Province (BE2016052) and the Innovation Project of Scientific Research and Practice for Postgraduates of Jiangsu Province is gratefully acknowledged.
†Trade name.
References
1 L.J. Fang, et al., “Cored-Wire Arc Spray Fabrication of Novel Aluminium-Copper Coatings for Anti-Corrosion/Fouling Hybrid Performances,” Surface and Coatings Technology 357 (2019): pp. 794-801.
2 M. Vostrák, et al., “Diagnostic of Laser Remelting of HVOF Sprayed Stellite Coatings Using an Infrared Camera,” Surface and Coatings Technology 318 (2017): pp. 360-364.
3 Y.Y. Zhang, et al., “Microstructural Analysis of Zr55Cu30Al10Ni5 Bulk Metallic Glasses by Laser Surface Remelting and Laser Solid Forming,” Intermetallics 66 (2015): pp. 22-30.
4 Q.Y. Wang, et al., “Effects of Laser Re-Melting and Annealing on Microstructure, Mechanical Property and Corrosion Resistance of FeBased Amorphous/Crystalline Composite Coating,” Materials Characterization 127 (2017): pp. 239-247.
5 J.B. Yu, et al., “Laser Remelting of PlasmaSprayed Nanostructured Al2O3-20 wt% ZrO2 Coatings onto 316L Stainless Steel,” Applied Surface Science 431 (2017): pp. 112-121.
6 J.N. Liu, et al., “Microstructure and Fatigue Damage Mechanism of Fe-Co-Ni-Al-Ti-Zr High-Entropy Alloy Film by Nanoscale Dynamic Mechanical Analysis,” Vacuum 159 (2018): pp. 516-523.
7 D. Zhang, et al., “Fabrication of Mg65Zn30Ca5 Amorphous Coating by Laser Remelting,” J. Non-Crystalline Solids 500 (2018): pp. 205-209.
8 G.Z. Xie, et al., “Effect of Laser Remelting on Corrosion Behavior of Plasma-Sprayed NiCoated WC Coatings,” Mater. Sci. Eng. 460 (2007): pp. 351-356.
9 G.Z. Xie, et al., “Corrosion Characteristics of Plasma-Sprayed Ni-Coated WC Coatings Comparison with Different Post-Treatment,” Corros. Sci. 49, 2 (2007): pp. 662-671.
10 N. Li, et al., “Corrosion Characteristics and Wear Performance of Cold Sprayed Coatings of Reinforced Al Deposited onto Friction Stir Welded AA2024-T3 Joints,” Surface and Coatings Technology 349 (2018): pp. 1,069-1,076