Metal corrosion can result in severe safety and environmental hazard problems.1 Corrosion-resistant coatings are considered to be an economical and effective method to protect metals from corrosion. However, traditional organic coatings do not perform well against corrosion due to inherent defects and low abrasion resistance.2
Epoxy powder coatings are widely used in corrosion-resistant coatings due to being environmentally friendly, having strong adhesion, and providing good electrical insulation.3 In fact, the electrical insulation properties of epoxy powder coatings are so good that underlying metal anodes can be shielded from cathodic protection when electrochemical corrosion occurs.
Researchers found that the permeability of the coating decreased and the corrosion protection thus improved after adding graphene nanosheets (GNS) into the epoxy coating.4-5 However, it is still a big challenge to provide proper dispersion of the GNS in the coating, which is key to proper corrosion resistance. Conductive polymers have been used to develop corrosion-resistant materials.6-7 Adding conductive polymers as pigments to epoxy coatings could effectively enhance the anticorrosion property of the coatings.8 Conductive polymers could also be used to develop the graphenebased nanocomposite to make the graphene well-dispersed into the coatings.9
Despite of the widespread use of the graphene/conductive polymer nanocomposite-reinforced organic epoxy coatings,10 not enough work has been done on the epoxy powder coating with graphene-doped polyaniline nanocomposite. In this article, a series of polyaniline/graphene/DBSA composites were prepared. The structure and morphology of the composite were characterized by Fourier transform infrared spectroscopy and scanning electron microscopy (SEM), respectively. The corrosion resistance of the samples with different doping ratios was tested by electrochemical impedance spectroscopy, polarization curves, and salt spray tests.
Results and Discussion
Morphological Characterization
The morphologies of the composites were characterized by SEM. From Figure 1, a large amount of polyaniline nanoparticles was “anchored” on the surface of GNS. The composite was loose and exhibited a finer sheet-like structure.
Structural Characterization
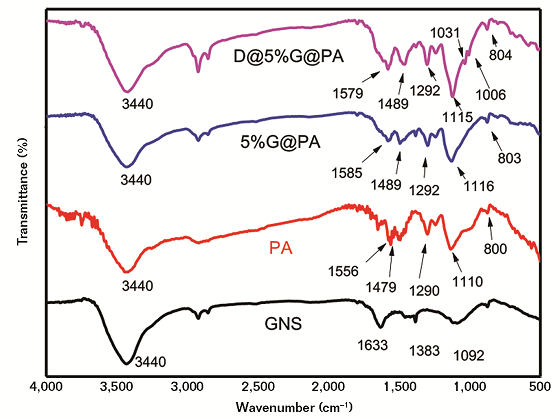
From Figure 2, the absorption peaks of GNS at 1,633 and 1,092 cm–1 belonged to the C=O stretching vibration and the C-O stretching vibration, respectively. The broad peaks at 3,440 cm–1 belonged to the O-H stretching vibration of hydroxyl groups. The stretching vibration of 2,922 cm–1 corresponded to the C-H bond and 1,383 cm–1 corresponded to the C-O bond of the epoxide group. The absorption peaks of PA at 1,556 and 1,479 cm–1 were the C=C stretching vibration peaks in the quinone structure (N=Q=N) and the C-C stretching vibration peaks in the benzene structure, respectively.11-12
The peak at 1,110 cm–1 was attributed to the C-N vibration absorption of the quinone structure and 1,290 cm–1 was the C-N stretching vibration characteristic absorption peak in benzene rings. Compared with the peaks of the PA, the corresponding peaks of the 5%G@PA composite had a red shift. These changes indicated the successful polymerization of aniline on GNS. The absorption peaks of D@5%G@PA composite appeared at 1,005 and 1,031 cm–1, which were characteristic peaks of the asymmetric stretching vibration frequency splitting of the aromatic sulfonic acid O=S=O, indicating that the Brönsted acid has been doped into the 5%G@PA composite.
Corrosion Resistance of the Coatings
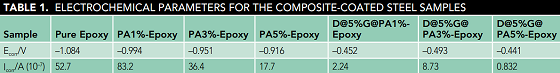
The corrosion resistance of the coating with pure epoxy, pure PA, and the composites were studied using an electrochemical workstation in a 3.5 wt% sodium chloride (NaCl) solution. Figure 3 and Table 1 show the polarization curve of the steel electrode. The Ecorr value of the pure epoxy resin was –1.084 mV. The Ecorr values of the epoxy coating with 1, 3, and 5 wt% pure PA were –0.994, –0.951, and –0.961 mV, respectively, a slight increase.
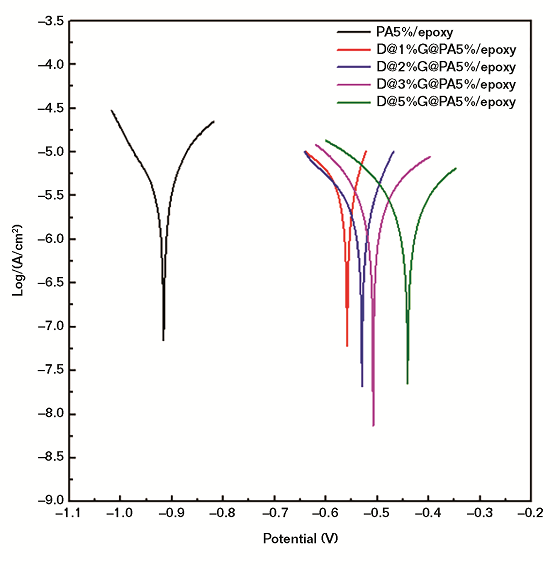
After adding the same weight of the DBSA@x%G@PA composites, the Ecorr value was increased significantly. For example, the Ecorr values of the epoxy coating containing 1, 3, and 5 wt% D@5%G@PA composites were –0.452, –0.493, and –0.441 mV, respectively. Compared with that of the coating with PA, the Icorr value of the coating with composite showed a significant decrease. The Icorr of the coating with 5 wt% PA was 1.77 × 10–6 A cm–2. However, the Icorr of the coating with the same weight of D@5%G@PA composite was as low as 8.32 × 10–8 A cm–2, indicating the epoxy coating with the composite showed better corrosion resistance. The probable reason was that the introduction of D@5%G@PA composite into the epoxy system made the coating more dense and increased the tortuosity of the diffusion path of oxygen and water.
Electrochemical Impedance Spectroscopy
In the electrochemical impedance spectroscopy (EIS) analysis of the steel substrate coated with pure epoxy, PA and the composite were performed at a 3.5 wt% NaCl solution. Figure 4 presents the EIS results. The value of the impedance modulus of the sample coated with epoxy resin at very low frequency (10 mHz) was 2.0 MΩ cm2 (Figure 4[a]). When 1 wt% PA was added into the epoxy resin, the value increased to 4.0 MΩ cm2 (Figure 4[a]).
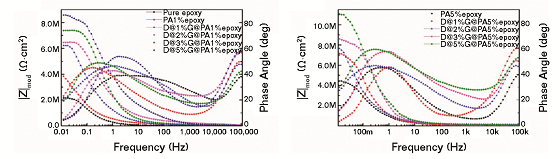
Based on the fact that the corrosion rate is inversely proportional to the value of impedance modulus at low frequency,13 it can be concluded that the epoxy resin with PA showed better corrosion resistance. The value of the impedance modulus of the epoxy with 1 wt% D@1%G@PA composite was 6.0 MΩ cm2 (Figure 4[a]), which is almost three times that of the epoxy resin, indicating a great improvement in the corrosion resistance. The enhanced corrosion resistance was probably attributed to the excellent barrier of the GNS toward corrosive species.
The value of the impedance modulus of the coating with 1 wt% D@2%G@PA, D@3%G@PA, and D@5%G@PA composite was 6.2, 7.5, and 8.5 MΩ cm2 (Figure 4[a]), respectively, suggesting that the corrosion resistance increased with increasing GNS. The value of the impedance modulus of composite coating with 5 wt% D@5%G@PA5 was 10.5 MΩ cm2 (Figure 4[b]), larger than that of the other composite coating, indicating an effective anticorrosion property.
Conclusions
A series of D@x%G@PA composite materials was prepared and characterized. The D@%G@PA composite was used as filler to prepare composite coatings. EIS result showed that the Icorr of the coating with 5 wt% D@5%G@PA composite was as low as 8.32 × 10–8 A cm–2. The value of the impedance modulus of epoxy coating with 5 wt% D@5%G@PA was 10.5 MΩ cm2, larger than that of the other composite coating, indicating the effective anticorrosion property. All of the samples coated with composite exhibited significantly lower corrosion rates than the epoxy sample.
Acknowledgments
The authors gratefully acknowledge the financial support of the National Nature Science Foundation (51303192), the Ningbo Major Special Project (2013B6012), and Scholarship of CSC (201808330007).
References and About the Authors