Corrosion damage to the outer surface of preheater tubes was discovered in the low-temperature zone of the heat recovery boiler at a power plant. The preheater unit was a horizontal, counterflow type heat exchanger and its tubes were used to preheat the water with the heat recovered from flue gases leaving the boiler. The flue gases were generated by burning the sulfur-bearing fuel, and the gases reached the preheater tubes at a temperature of 160 °C. During operation, the average surface temperature of the preheater tubes was 120 °C. The 38-mm (1.5-in) diameter, 2.39-mm (0.094-in) thick tubes were made of carbon steel (CS) grade ASTM SA-178A, and had been in service for five years. The primary goal of this analysis was to determine the causes of the damage to the CS preheater tubes.
Visual Observation
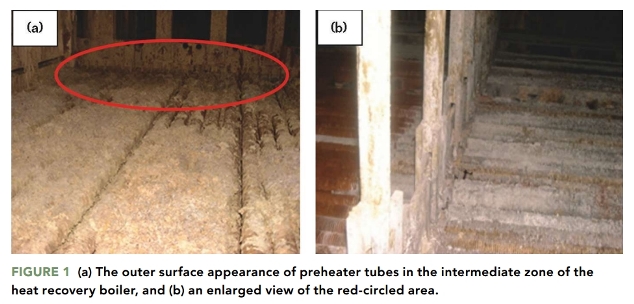
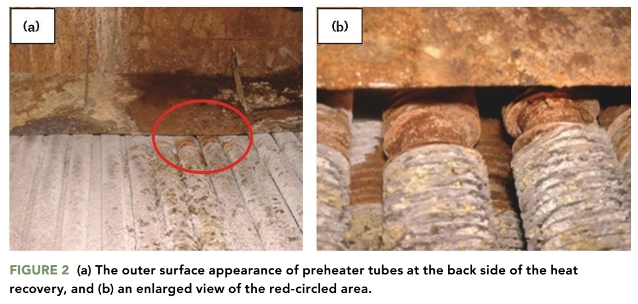
Figures 1 and 2 show the outer surface appearance of the preheater tubes in the intermediate zone and at the back side of the heat recovery boiler. The outer surface of the preheater tubes in both areas was covered with corrosion products, indicating that both areas were subjected to external corrosion caused by exposure to the environment. There is a relationship between the color and composition of the corrosion products.
The corrosion product color in Figures 1 and 2 is white-yellow. This may indicate a corrosion reaction between the steel and sulfur-containing environment, which resulted in the formation of ferrous/ferric sulfate on the outer surface of the preheater tube.1-2
Localized corrosion attack was also observed at a weldment, as shown in Figure 3. This suggests that the welding region is the preferential site of attack. These visual findings show that the fuel gas environment in the low-temperature zone of the heat recovery boiler is heavily corrosive to preheater tubes.
Corrosion Investigation Analysis and Discussion
The accumulation of corrosion product layers on the outer surface of the preheater tubes indicates that the gas from the burned fuel is possibly a leading cause of this corrosion damage. Sulfur and moisture in the fuel play an important role in this corrosion failure. Their reaction results in the formation of free acid, which promotes the corrosion attack.3 The surface temperature of the tubes is related to the condensation of acidic gases in the flue gas. Where condensation occurs, an electrochemical corrosion cell is established. These are considered to be the three corrosion-influencing factors in the environment to which the preheater tubes are subjected.
The Role of Sulfur and Moisture
Sulfur and its derivatives are known corrosion stimulators for steel, particularly in the form of sulfuric acid (H2SO4). Fuel containing a high amount of sulfur can cause steel corrosion problems, as reported by many authors.4 When fuels containing sulfur are fired, sulfur is oxidized to sulfur dioxide (SO2), which can be further oxidized to sulfur trioxide (SO3), as described in Equation (1).5-6
2SO2 + O2 → 2SO3 (1)
If moisture is present in the environment, it will then react with SO3 to produce H2SO4 vapor, as detailed in Equation (2).
SO3 (g) + H2O → H2SO4 (2)
This acid vapor can be dissociated to SO3 and water in high-temperature conditions. At a low temperature, however, H2SO4 vapor can condense and form liquid H2SO4.7
The Role of the Surface Temperature
The surface temperature of the preheater tubes defines where condensation of H2SO4-bearing gases will occur. If the surface temperature of the preheater tubes is sufficiently low, the acidic gases can condense. When a condensed acidic electrolyte is formed, the corrosion process begins.
Corrosion Damage Mechanism
Generally, corrosion damage of a metal is due to the interaction between the metal and its environment. In the case of the corroded preheater tubes, the combustion of fuel emits an acidic precursor gas, SO2, that can be oxidized to form SO3. The presence of moisture in flue gas allows conversion of SO3 into H2SO4 vapors. When the temperature of the outer surface of the tube is low, an acidic electrolyte can be formed, especially when the temperature is lower than the dew point temperature of H2SO4 vapor. As the H2SO4-containing electrolyte covers the tube surface, it can corrode the steel, as shown in Equation (3).8
Fe + H2SO4 → FeSO4 + H2 (3)
Ferrous sulfate (FeSO4) is known to be a major component of the corrosion products that cover the steel exposed to sulfur-containing environments.9 The white-yellow corrosion product observed in Figures 1 and 2 indicates the presence of FeSO4.
Conclusions and Recommendations

Based on the corrosion analysis of preheater tubes located in the low-temperature zone of the heat recovery boiler, it can be concluded that the preheater tubes were attacked by the condensed H2SO4-containing electrolyte. The combustion of fuel introduced SO3 to the environment where the tubes were exposed. The moisture present in the combustion product facilitated conversion of SO3 to H2SO4 vapor. The low temperature of the tube surface enabled the formation of the H2SO4-containing electrolyte by condensation. This condensed electrolyte, which was very acidic in nature, then attacked most of the steel tubes in the preheater unit, as illustrated in Figures 1 and 2. The preferential site of this attack was the weldment of tubes, as shown in Figure 3.
The corrosion problem could be minimized by using a better quality of fuel with lower sulfur and moisture content. The condensation of an acidic electrolyte can be avoided by keeping the metal temperature of the preheater tubes above the dew point of H2SO4 vapor.
References
1 R.D. Port, H.M. Herro, The Nalco Guide to Boiler Failure Analysis (New York, NY: McGraw-Hill, 1991).
2 Y. Gong, Z.G. Yang, “Corrosion Evaluation of One Dry Desulfurization Equipment—Circulating Fluidized Bed Boiler,” Materials and Design 32 (2011): pp. 671-681.
3 V. Ganapathy, “Cold-end Corrosion: Causes and Cures,” Hydrocarbon Processing 1 (1989): pp. 57-59.
4 C. Jin, H. Chen, L. Wang, X. Zhang, “Corrosion Analysis of the Steel Structure Surrounding a Coal-Fired Boiler Heating Plant,” MP 54, 1 (2015): pp. 72-74.
5 A.U. Malik, S.A. Al-Fozan, M. Mobin, M. AlHajri, “Studies on the Failure of Economizer Tubes Involving Acid Dew Point Corrosion in High Pressure Boiler,” Intl. J. of Scientific & Engineering Research 4, 1 (2013): pp. 1,726-1,736.
6 F. Gabriella, “An Overview of Water Related Tube Failures in Industrial Boilers,” MP 27, 6 (1988): p. 51.
7 L.L. Shreir, R.A. Jarman, G.T. Burstein, Corrosion, Vol. 1, “Metal/Environment Reaction,” 3rd ed. (Oxford, U.K.: Butterworth Heinemann, 2000).
8 G. Kreysa, M. Schutz, Corrosion Handbook— Corrosive Agents and Their Interaction with Materials, Vol. 6, “Atmosphere, Industrial Waste Gases” (Frankfort, Germany: DECHEMA, 2006).
9 R.W. Revie, H.H. Uhlig, Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 3rd ed. (Hoboken, NJ: John Wiley & Sons, Inc., 1991).