The corrosion of pre-stressing strands can have disastrous consequences if not detected early. Recently in 2016, a tendon failure on the Wando River bridge in Charleston, South Carolina, USA resulted in subsequent damage of nearby structural components.1
Metallurgical analysis performed by Applied Technical Services2 reported that the failure was the result of loss in cross-sectional area and loss in flexibility due to corrosion. The authors stated that moisture ingress must be present to produce the localized corrosion observed.
Analysis of the steel strands performed by the University of South Florida showed that some of the failed steel strands exhibited brittle-like fracture, possibly the result of hydrogen adsorption. Hydrogen content analysis performed by Luvak Inc. in Boylston, Massachusetts, USA reported
that some of the steel strands had absorbed as much as 69 ppm of diffusible hydrogen determined by vacuum pressure testing (inactive ASTM E146-83 3) indicating that hydrogen embrittlement (HE) could be of concern.4
A review is presented focused on the conditions that promote hydrogen production and the mechanisms of HE of cold-drawn pearlitic prestressing steels in an effort to determine the implications of grout conditions within galvanized steel ducts to prestressing steel failures.
Proposed Mechanisms of Hydrogen Embrittlement
Johnson reported the decrease in the mechanical properties of metals due to the presence of hydrogen in 1875,5 yet the mechanisms involved are still under investigation. Hydrogen molecules may be produced by hydrogen evolution because of the cathodic processes associated with the corrosion reaction or be driven electrochemically during cathodic polarization.6
In the first stage of hydrogen evolution, hydrogen atoms adsorb on the metal’s surface by discharging hydrated protons or water splitting. Then, in the second stage, depending on the metal and the current density, the adsorbed hydrogen may desorb and form hydrogen molecules. Only a small amount of the adsorbed hydrogen is usually absorbed by the metal.7 Once hydrogen is absorbed into the metal, it will either diffuse through the lattice through interstitial sites or become trapped by lattice defects.
At lower energy trap sites, the hydrogen may subsequently break free and continue to diffuse through the lattice structure. If the barrier energy is high, the hydrogen is said to be irreversibly trapped.
Several theories have been proposed to describe HE of high-strength low-alloy steels (HSLA) but hydrogen-enhanced decohesion (HEDE) and hydrogenenhanced localized plasticity (HELP) are believed to be the primary embrittlement mechanisms. HEDE was introduced in 1926 by Pfeil et al.8 According to their theory, H decreases the cohesive metallic interatomic interactions and, hence, atoms become separated under low tensile stresses.
Increasing H concentration will lead to decreasing of the metallic interatomic forces. This mechanism is mainly proposed for HSLA steels with high levels of hydrogen concentration. Impurities in the grain boundaries, such as phosphor or sulfur, also will cause intergranular decohesion, which promotes further hydrogen attraction and will accelerate the fracture. In 1972, by careful study of hydrogen-assisted fracture surfaces, Beachem et al. 9 noticed tear ridges on brittle fracture surfaces and presented the HELP mechanism.
In this mechanism, H accelerates dislocation formation and motion, resulting in local dislocation pileups with early failure of the material. This mechanism is based on the interaction between the H and dislocations. It was described to realize the effect of plasticity (because of dislocation motion) on the fractured samples.
For pearlitic steels, HELP and HEDE are believed to be the primary embrittlement mechanisms. To determine whether HELP or HEDE is the major mechanism in HS steels, a micromechanical model of the fracture was developed by Toribio et al.10 Figure 1 shows the micromechanical model of HE in slightly cold drawn (CD) steels with a tearing topography surface (TTS).
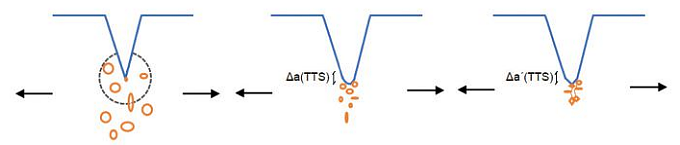 This tearing mode can be described by the HELP mechanism and some degrees of plasticity. The appearance of the TTS mode looks like a ductile process similar to fatigue crack propagation, and it has been verified that the main crack growth mechanism during cyclic loading is plasticity-induced transfer of material from the crack tip to the apex.10
This tearing mode can be described by the HELP mechanism and some degrees of plasticity. The appearance of the TTS mode looks like a ductile process similar to fatigue crack propagation, and it has been verified that the main crack growth mechanism during cyclic loading is plasticity-induced transfer of material from the crack tip to the apex.10
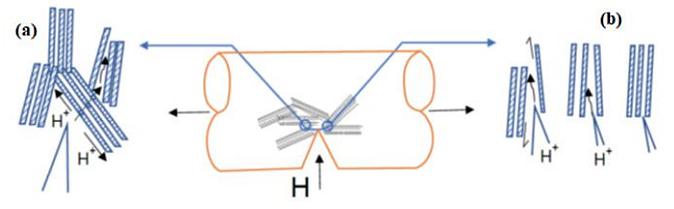
Figure 2 shows the micromechanical model of HE in HS steels with an intermediate degree of CD. Hydrogen diffuses at first inside the pearlitic microstructure. Then, hydrogen assisted cracking (HAC) happens in the form of either trans-lamellar fracture path (HELP by TTS) or by HEDE.
During HE in heavily CD steels, hydrogen at first diffuses through the preferential path parallel to the ferrite/cementite lamellae. Then, HAC occurs according to the HEDE mechanism. Factors causing the HE of CD steel resulting in a mixed elastic/plastic fracture mode were also investigated from the perspective of lattice defects. It was revealed that the number of lattice defects will not increase in the presence of H and under applying elastic stress and, hence, they are not a contributing factor to HE of CD pearlitic steel fractures in the elastic region, while they are the cause of the HE of CD steel that fractures in the plastic region.11
 Relationships Between Hydrogen Content and Mechanical Properties
Relationships Between Hydrogen Content and Mechanical Properties
While it is very difficult to relate the amount of absorbed hydrogen to its influence on macro-level mechanical properties, many attempts to do so have been made for a range of materials considering the discussed mechanisms of embrittlement. One such model12 was developed for HS pearlitic steels and provides an estimate of the flow stress, which decreases as a function of the effective concentration of H involved during decohesion at a given strain. The model requires a fitting parameter that can be determined from experimental results.
Additionally, Enos et al.13-14 performed a series of constant-extension-tensile tests (CERT) and introduced a general relationship between diffusible H concentration (in lattice + weakly trapped) (CH) and the local fracture-initiation stress:
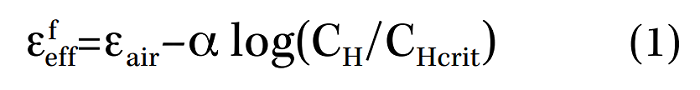 The general criticism is that many of the models relating mechanical properties to hydrogen content are based on a rate-limiting process and do not consider specific qualities of the microstructure that may influence crack growth or fracture stress.
The general criticism is that many of the models relating mechanical properties to hydrogen content are based on a rate-limiting process and do not consider specific qualities of the microstructure that may influence crack growth or fracture stress.
Implications to Grouted Post-Tensioned Tendons
Structures incorporating post-tensioned tendons are expected to have a service life of many decades, but often need rehabilitation within several years of service due to corrosion. Corrosion, in many cases, has occurred in regions of grout deficiencies in the form of voids formed by bleed water accumulation and subsequent reabsorption or evaporation through incomplete anchorage sealing.
Other possible causes of corrosion include intrusion of external chloride and water through anchorages or defects in the HDPE and possible adverse galvanic coupling between anchorage components and the strand. In some cases, corrosion of the steel may be accompanied by hydrogen absorption and subsequent embrittlement resulting in brittle failure.15
In establishing the susceptibility of a system to HE, the sources of hydrogen, its absorption and transport rates, and the influence on mechanical performance need to be identified. According to the reported failures, voided tendon ducts seem to play a primary role in the failure modes. While hydrogen is not usually present in the environment within post-tensioned ducts, hydrogen uptake by prestressing steels in voided regions has been reported.
Fernandez et al.15 measured the amount of hydrogen uptake by stressed steels in voided ducts reflecting the construction period following placement of the strands and prior to grout placement, which in some cases can be a couple of weeks. Strands contained within closed voided ducts with water showed a much higher hydrogen content than strands contained within closed dry ducts.
Additionally, Hartt et al.16 showed that within galvanized steel ducts, as used in the Wando River Bridge at the site of failure, hydrogen is cathodically produced at the strand surface due to galvanic coupling to the galvanized duct immediately following grouting.
The authors suggested that enough hydrogen is produced during this time to potentially cause embrittlement. However, the amount of hydrogen that was absorbed into the steel during the period immediately following grouting was not measured. Immediately following placement of the grout, the grout is wet and has a high conductivity, which promotes galvanic actions between the galvanized duct and the steel strands. As the grout cured, the coupling diminished. Based on this information, the question arises of what may occur if aggressive water infiltrates the galvanized steel tendon duct, potentially resulting in a highly conductive grout.
If conditions are present that promote hydrogen production, even if the absorbed hydrogen does not lead to substantial material embrittlement, it may have an influence on the corrosion resistance of the material. Absorbed hydrogen has been shown to influence the stability of the passive film and result in increased corrosion rates.17
On passive metals, the H atoms diffuse through the passive film, reduce the O2- ions to OH- ions, and OH- ions to water (H2O) molecules. These products may exchange with the Cl- ions, if present, and destabilize the passive film. Also, the presence of hydrogen may cause a reduction in the stability of the passive film and may result in a decrease in its thickness.
In non-passivating conditions, adsorbed hydrogen can also increase the corrosion rate of steels. The potential impact of hydrogen on corrosion rate of these steels may be influenced by several factors including decohesion of metallic bonding, increasing mobility of dislocations, hydrogen-induced phase transformation, and formation of vacancies in the metallic lattice.18
Summary
The primary mechanisms of HE of cold-drawn pearlitic steels include HEDE and HELP. A micromechanical model may be used to identify which mechanism controls at given levels of strain, but for a given failure due to HE both mechanisms may occur.
While relationships have been developed to describe the change in mechanical properties due to hydrogen absorption and diffusion of steels in general, few have been proposed specifically for cold-drawn pearlitic steels. An empirical model based on the results of the CERT test suggests a logarithmic relationship between diffusible hydrogen concentration and the local fracture-initiation strain.
Prior evidence shows the possibility of galvanic coupling between steel and galvanized structural components could promote hydrogen production. Therefore, high conductivity grout, either a result of improper mixing or water intrusion, may promote HE.
In the case that voids are present in the grout leaving the steel strand unprotected, hydrogen absorption could occur through corrosion processes or previously absorbed hydrogen could enhance corrosion activity. Further work is required to quantify the potential influence of HE combined with corrosion on the mechanical properties of cold-drawn pearlitic steels.