Bridges are designed to meet or exceed a predetermined service life, normally around 75 years. This lifespan can be difficult to meet when these bridges are located in a marine environment and contain cracks in the concrete. In such conditions, chloride-induced corrosion occurs, often at a rapid rate.
Researchers Margareth Dugarte and AMPP member Alberto A. Sagüés performed long-term studies to ascertain the effectiveness of cathodic protection (CP) to protect marine bridges.1 These tests, which spanned 5.5 years, studied reinforced concrete blocks to which they added controlled-width cracks of various sizes along the central reinforcing steel bar. Exposure to sodium chloride (NaCl) was accomplished through ponding and different polarization levels were utilized. Additionally, certain samples were autopsied, and final results of the study were analyzed to determine the effectiveness of CP under the different conditions.
The scientists emphasize that improving the quality of the concrete used in creating marine structures can be accomplished by making it less permeable and increasing the clear cover. When these two steps are taken, the time to corrosion is significantly longer.
Unfortunately, they can also come with drawbacks, including cost and special handling requirements to prevent cracking.
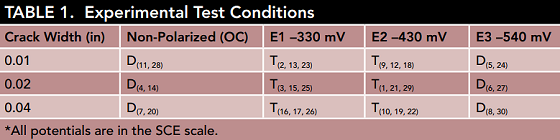
For the experiment, 30 concrete prisms containing three embedded reinforcement bars were built. The team created cracks at specific widths in each sample through the use of three-point bending and metal shims. The width of the cracks is specified in Table 1. Cracks are known to cause extreme issues in regard to durability since they allow chloride ions to access the reinforcement more easily. Higher chloride concentrations have been found at the steel/concrete interface of low permeability concrete with cracks compared to that of sound concrete. Consequently, in high-performance concretes, early corrosion is most likely to be seen at crack sites.
Next, to simulate a marine environment, the researchers utilized ponding in which the specimens were subjected to a 5% NaCl solution either cyclically or continually. Polarization levels between –430 to –640 mV vs. the saturated calomel electrode were applied. Note that the embedded reinforcement bars were typically interconnected, and polarization was applied concurrently in the cathodic prevention (Cprev) system. This was accomplished through the use of an MMO activated titanium mesh ribbon anode that was attached to a multichannel potentiostat (Figure 1).
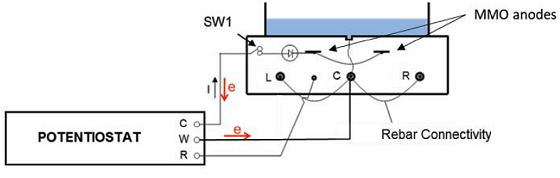
Engineers are increasingly focused on durability and corrosion control when designing current structures, including methods like CP and Cprev. In the case of cracked concrete, traditional CP is worth considering as its main benefit is the negative shift in potential. This can lower the risk of anodic reaction and increase the resistance to the anodic process. While CP is effective, high current densities may be needed—often 2 to 20 mA/m2 on existing structures and 2 to 5 mA/m2 for new structures.
In cases where corrosion initiation is delayed due to elevation of the chloride threshold at more negative potentials in Cprev, less polarization is necessary. In the instance of previously cracked concrete, the use of Cprev might prove difficult because high local chloride contents probably exist on the steel surface at the intersection of the cracks.
In addition to the crack widths used in the experiment, Table 1 also provides the potential level for each specimen and the degree of replication for each condition. The scientists emphasize that “OC” refers to the interconnected three-bar assembly, which was not connected to an external current source—rather macro-cell currents flowed between the bars. For the OC specimens, the point of sudden potential drop is when they were deemed activated, and for the polarized specimens, it was determined by two conditions; first, once the total cathodic current demand of the three-rebar group disappeared, and second, once the central bar’s current became anodic.
When the specimens were autopsied, a corrosion influence zone was found at crack-steel intersections. Mass loss calculations were then performed to determine the amount of corrosion. Figure 2 (top) shows one of the specimens after the autopsy in which the center bar showed corrosion products at the crack intersection. The two sidebars had no corrosion as predicted.
Results of the study revealed several important points. Without protection in a simulated marine environment, such as CP or Cprev, the reinforcing steel in cracked concrete actively corroded after only a week of exposure. An ambitious service life goal would be difficult to achieve in the segment of the bridge containing the crack(s).
Additionally, testing the efficacy of CP and Cprev on concrete that was cracked lengthwise along the steel bar concluded that polarization at potentials of –330 and –430 mV offered greater time to activation regarding corrosion. The increase was found to be longer in narrower crack sizes, but the researchers stress that on all crack widths tested during the 5.5-year study, the polarization levels were unsuccessful at preventing corrosion.
And finally, although not entirely effective at corrosion prevention over the long term in areas with cracks, once the polarization arrived at –540 mV, the time to corrosion initiation did increase.
Reference
1 M. Dugarte, A.A. Sagüés, “Cathodic Prevention/Protection of Marine Corrosion of Steel in Previously Cracked Concrete,” CORROSION 2021, paper no. 16952 (Houston, TX: NACE International, 2021).