Abrasives are critical for conditioning the surface to which a coating is applied, and their cleanliness helps achieve the specified surface requirements. A wealth of technically sound documentation exists showing that soluble salts left on a surface are a leading cause of coating failure.1-8
To refine or confirm the cautionary limit for chloride and sulfate soluble salt species on abrasives, commonly used commercial abrasives were tested, with the results documented by a NACE-certified Coating Inspector.
Test Procedures
The testing criteria limited work to commonly used abrasives that achieve a 0.050 to 0.076-mm (2 to 3-mil) profile on carbon steel (CS), and the abrasive blast hose pressure of 0.66 MPa (95 lb/in2). A commercial compressor, a six-bag abrasive pot, and an abrasive nozzle were used for the test. Most of the abrasives used for this testing were of a 0.60/0.25 mm or 0.85/0.30 mm (30/60 or 20/50 mesh) blend.
All CS panels used had previously been automated-wheel blasted with steel grit to remove mill scale and rust to establish consistent surfaces for each blast test and were then placed in sealed plastic bags with desiccant packages. The steel grit had been tested prior to abrasive blasting to ensure compliance with SSPC AB-2.9

Prior to blasting with the test abrasives:
• All panels were tested for any residual chloride, sulfate, and nitrate. Levels were found to be nondetectable in all cases (Figure 1).
• Samples from the top and bottom of bags of the selected abrasives were tested for levels of chloride, sulfate, and nitrate to establish consistency within a lot or bag quantity (Figure 2).
 Each sampled material was used to abrasive-blast dedicated steel panels (Figure 3), and cross-contamination was prevented by starting with a clean abrasive pot, with oil/water separators and air dryers, and cleared lines with each abrasive used. The ASTM D428510 standard test method for indicating oil or water in compressed air was performed prior to each test to ensure there was no cross-contamination from this potential source.
Each sampled material was used to abrasive-blast dedicated steel panels (Figure 3), and cross-contamination was prevented by starting with a clean abrasive pot, with oil/water separators and air dryers, and cleared lines with each abrasive used. The ASTM D428510 standard test method for indicating oil or water in compressed air was performed prior to each test to ensure there was no cross-contamination from this potential source.
Immediately thereafter, the blasted panels were tested for any transferred soluble chloride, sulfate, and nitrate anionic species. All the testing was conducted on the same day in the low-humidity environment of Bakersfield, California. The results are shown in Table 1.
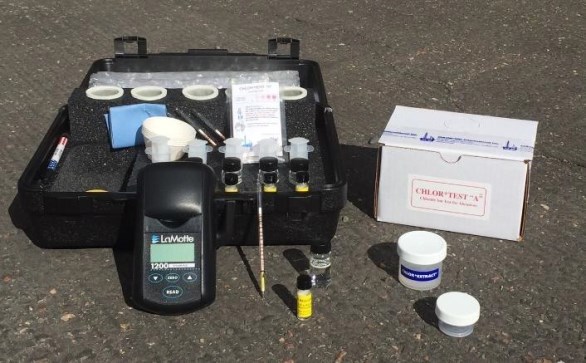
Testing of both surfaces and materials was conducted with the accurate CHLOR*TEST "CSN"† field test kit (Figure 4), the test kit referenced in SSPC Guide 15.11 Abrasive samples were prepared using the CHLOR*TEST "A"† field kit, which follows the standard for sample preparation outlined in ASTM D4940.12 Figure 5 shows measured test levels for chlorides, sulfates, and nitrates.
This represents a small but meaningful spot check of commercial abrasives used commonly for surface preparation. Conditions of pressure, particle size, friability, and hardness can all contribute to transfer levels. The results in Table 1 can be compared with those in Table 2, which were from small samples of various commercially used abrasives received two months prior and were analyzed for chloride, sulfate, and nitrate contamination with the same field test kit method outlined previously.

The test results in Table 1 can be compared with prior published tests using doped abrasives to certain chloride contamination levels to determine transfer rates and in order to provide some initial guidance on limits, which may be considered “safe” for not transferring soluble anionic salt species to the substrate. These include:
• Research conducted at Transport SA (Adelaide, South Australia) calculated a limit of ~20 mg/kg (ppm) of chloride before transfer to blasted substrates occurs.13
• A European Commission report was interpreted and extrapolated to reflect a level <20 mg/kg (ppm) of chloride as a limit before transfer occurred.14
A further data point is the limit reflected in the 11127 series of ISO standards, which specify a limit of 25 mg/kg (ppm) of chloride from various abrasives. No details were found to validate the basis for this data.
Conclusions
The following facts are summarized from the data collected:
• Relative to transfer of soluble anionic species, the sulfate anion does not transfer as readily as chloride. This may be because soluble sulfate is more strongly adsorbed to the abrasive particles than chloride.
• The use of conductivity, based on the above, can lead to the rejection of usable material. Higher levels of soluble sulfate generate higher conductivity yet do not demonstrate the same rate of transfer.
• Variations in levels of soluble salts between samples would indicate a need for caution in abrasive selection, especially for surfaces that will be subjected to critical service.
• The wide variation in levels addressed in industry standards may not be taking into account the evolving and more stringent limits to achieve the requirements of full lifecycle coating performance, especially in critical or severe service.
• A statistically significant data set developed from multiple blasted test panels could be used to generate criteria that meet the desired stringent requirements of surface cleanliness prior to coating.
†Trade name.
References
1 H.H. Uhlig, W.R. Revie, Corrosion and Corrosion Control, 3rd ed. (New York, NY: John Wiley and Sons, Inc., 1985), pp. 74-78.
2 B.R. Appleman, “Painting Over Soluble Salts: A Perspective,” JPCL 4, 10 (1987): p. 68.
3 B.R. Appleman, et al., “Effects of Surface Contaminants on Coating Life,” SSPC/FHWA, SSPC 91-07, April 1992.
4 M. Morcillo, J. Simancas, “Effects of Soluble Salts on Coating Life in Atmospheric Service,” JPCL 14, 9 (1997): p. 40.
5 M. Morcillo, F.J. Rodriguez, J.M. Bastides, “The Influence of Chlorides, Sulphates, and Nitrates at the Coating-Steel Interface on Underfilm Corrosion,” Progress in Organic Coatings 31 (1997): pp. 245-253.
6 H. Mitschke, “Effects of Chloride Contamination on the Performance of Tank and Vessel Linings,” JPCL 18, 3 (2001): p. 49.
7 NACE Publication 6G186: “Surface Preparation of Soluble Salt Contaminated Steel Substrates Prior to Coating” (Houston, TX: NACE International, 2010).
8 ISO TR 15235, “Preparation of steel substrates before application of paints and related products—Collected information on the effects of levels of water-soluble salt contamination” (Geneva, Switzerland: ISO, 2001).
9 SSPC-AB 2, “Cleanliness of Recycled Ferrous Metallic Abrasive” (Pittsburgh, PA: SSPC, 2016).
10 ASTM D4285-83 (2012), “Standard Test Method for Indicating Oil or Water in Compressed Air” (West Conshohocken, PA: ASTM, 2012).
11 SSPC Guide 15, “Retrieval and Analysis of Soluble Salts” (Pittsburgh, PA: SSPC, 2013).
12 ASTM D4940-15e1, “Standard Test Method for Conductimetric Analysis of Water Soluble Ionic Contamination of Blast Cleaning Abrasives” (West Conshohocken, PA: ASTM, 2015).
13 D.M. Richards, “Effects of Chloride Contamination of Abrasives on Performance of Long Life Coatings of Steel,” Coatings for Asia 99, Singapore (1999): pp. 181-191.
14 M. Bohm, et al., “Soluble Salt Contamination on Blasted-Cleaned Surfaces and the Effect on the Durability of Subsequently Applied Coatings—Final Report,” Technical Steel Research, Directorate General for Research (Luxembourg, European Commission, 2005), pp. 49-50.