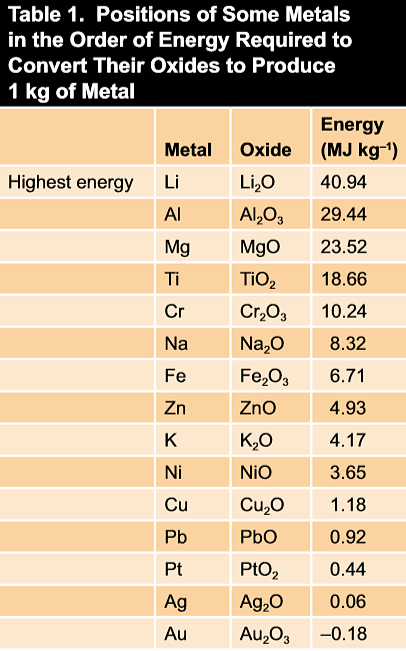
The driving force that causes metals to corrode is a natural consequence of their temporary existence in metallic form. To reach this metallic state from their occurrence in nature in the form of various compounds (ores), it is necessary for them to absorb and store up the energy required to release the metals from their original compounds for later return by corrosion. The amount of energy required and stored varies from metal to metal. It is relatively high for metals such as magnesium, aluminum, and iron, and relatively low for metals such as copper, silver, and gold. Table 1 lists a few metals in order of diminishing amounts of energy required to convert them from their oxides to metal.
A typical cycle is illustrated by iron. The most common iron ore, hematite, is an oxide of iron (Fe2O3). The most common product of the corrosion of iron— rust—has a similar chemical composition. The energy required to convert iron ore to metallic iron is returned when the iron corrodes to form the original compound. Only the rate of energy change may be different.
The energy difference between metals and their ores can be expressed in electrical terms that are related to formation heats of the compounds. The difficulty of extracting metals from their ores in terms of the energy required, and the consequent tendency to release this energy by corrosion, is reflected by the relative positions of pure metals in a list.
Destruction by corrosion takes many forms and depends on the complex interaction of a multitude of factors, such as:
 Nature of the metal or alloy.
Nature of the metal or alloy.- Presence of inclusions or other foreign matter at the surface.
- Homogeneity of the metallic structure.
- Nature of the corrosive environment.
- Incidental environmental factors such as the presence of oxygen and its uniformity, temperature, and velocity of movement.
- Stress (residual or applied, steady or cyclic).
- Oxide scales (continuous or broken).
- Presence of porous or semiporous deposits on surfaces, built-in crevices.
- Galvanic effects among dissimilar metals.
- Occasional presence of stray electrical currents from external sources.
Some environments are more corrosive than others. Except in rare cases of a grossly improper choice of material for a particular service, or an unanticipated drastic change in the corrosive nature of the environment or complete misunderstanding of its nature, failures of metals by rapid general attack (wasting away) are not often encountered. Corrosion failures are more often subtle and a result of invisible localized effects in the form of pits, intergranular corrosion, or attack within crevices.
This article is adapted from Corrosion Basics—An Introduction, Second Edition, Pierre R. Roberge, ed. (Houston, TX: NACE International, 2006), pp. 21-22.