Microorganisms—they have been around for billions of years and can live in incredibly difficult environments and under extremely severe conditions. They utilize exceptionally diverse food sources and some of the byproducts produced by their metabolisms can be damaging to metals. The corrosion of a material when the presence of microorganisms plays a role in is known as microbiologically influenced corrosion (MIC). Activities of bacteria, Archaea, and fungi in colonies that create biofilms on surfaces of materials, or in local environments that directly contact materials, can result in MIC; and most metals, as well as some nonmetals, can be affected by this type of corrosion.
According to NACE standard TM0212-2012,1 which focuses on detecting, testing, and evaluating MIC on internal pipeline surfaces, MIC typically takes place in the presence of a conglomerate of microbes comprised of multiple types of microorganisms. Depending on the environment, these microbes may include metal-oxidizing bacteria, sulfate-reducing bacteria (SRB), acid-producing bacteria (APB), metal-reducing bacteria (MRB), and methanogens. The presence of microorganisms may create conditions that foster corrosion initiation, or their metabolic reactions may maintain conditions that promote continued corrosion. MIC does not produce visually unique corrosion morphology. Instead, MIC often results in pitting, crevice, underdeposit, and galvanic corrosion, as well as dealloying.

One of the most profound challenges that corrosion professionals face when trying to identify MIC is making a connection between the microorganisms present in a system and the corrosion experienced by that system, says NACE International member Richard B. Eckert, senior principal engineer—corrosion management, Materials Advisory Services, with DNV GL—North America Oil & Gas. In the oil and gas industry, microorganisms can be found in nearly every oil and gas production environment, especially pipelines. They inhabit the bulk fluids that flow through oil and gas pipelines, and also form biofilms on the internal surfaces of pipes. Eckert, who serves as chair of NACE Task Group (TG) 254, which published TM0212-2012, comments that the presence of microorganisms doesn’t necessarily mean they are the cause of localized corrosion. In any environment, he says, there are usually multiple factors that contribute to corrosion, and different corrosion mechanisms, including MIC, can result in the same corrosion morphology. This is why it’s crucial to verify there is a clear relationship between the biofilm and corrosion, he stresses. For example, corrosion could be caused by an abiotic reaction fueled by the presence of water and oxygen that doesn’t involve microorganisms at all, even though they are present.

There are several objectives for determining the characteristics of a microbiological presence where corrosion is found. These include establishing a connection between microbiological activities and corrosion reactions and corrosion products in a particular environment; identifying specific microbes that support the corrosion mechanism seen in that environment; and associating the corrosion reactions/corrosion damage with the presence of microbes at some point in the corrosion process.2 TM0212-2012 states that three conditions need to be met to validate MIC as the cause of internal corrosion.
-
Assuming there is no known or suspected contamination from outside sources, the first condition is a demonstration of increased levels of specific types of viable microorganisms (bacteria or fungi) associated with the corrosion, as compared to samples taken outside of the corroded area.
- The second condition is the identification of
chemical indicators in the corroded area that support the microbiological
evidence (e.g., elevated levels of sulfide or sulfur in pit deposits for SRB,
or organic acids for APB).
- The third
condition is the identification of biotic factors as the primary contributor to
the corrosion damage. The nature of the corrosion damage to the pipeline system
should be consistent with the nature of the identified microorganism(s) and
their byproducts or physical influence on corrosion cell formation. For
example, if viable APB or methanogens are concentrated at the corrosion damage
relative to the environment, and evidence of their metabolic activity (organic
acids) is determined to be associated with the corrosion, the nature of the
corrosion damage should be consistent with these observations (e.g.,
accelerated corrosion damage or pitting beneath the biofilms). This is an
important step in a diagnosis of MIC because it is often difficult to discern
between the relative contributions of various biotic and abiotic factors that
affect localized corrosion.
To properly diagnose MIC, investigations should comprise a combination of chemical, metallurgical, and microbiological analyses. TM0212-2012 presents information on sample collection, testing methods, corrosion monitoring, and how to relate the results to MIC.
Sampling
Sampling programs generally collect information on operating conditions, corrosion rates, and microbiological conditions over a period of time so any trends in the findings can be identified. Also, due to normal statistical variations associated with sample collection and microbiological testing, more reliable data typically result from performing tests on samples representing a time range rather than any single sample. Data collection and analysis should focus on differentiating the effects of biotic and abiotic factors on the likelihood, location, cause, and severity of internal corrosion.
Samples of bulk fluids from pipelines are often collected to identify and quantify levels of planktonic (moving) microorganisms. To ensure that test results are relevant to MIC, data from bulk fluids should be correlated with other pipeline data, including liquid composition, operational conditions, sessile (attached) microorganism counts, and corrosion data. Since both corrosion and microbiological activity may occur directly on the internal surfaces of a pipeline, samples that are representative of these surfaces may provide significant information and should be collected, whenever possible, in conjunction with each liquid sample retrieved. Eckert notes that it can be very difficult to infer what is happening on the pipeline’s internal surface based solely on the composition of the bulk fluid because there isn’t always a clear relationship between what is found on the surface and what comprises the bulk fluid phase. The microorganisms living in the bulk fluid (types and quantities of microbes), and even the chemical composition of the bulk fluid, can be far different than what is found on the internal pipe surface—that’s been well established in research, he adds.
Microbiological Testing
Microorganisms are highly sensitive to environmental changes that affect nutrient availability, flow rates, temperature, salinity, the presence of dissolved gases, etc. Additionally, many chemicals produced by microbial metabolisms (e.g., organic acids and sulfide compounds) can be quickly oxidized or degraded by environmental changes. Because conditions can change rapidly once a sample is removed from the pipeline, certain tests relevant to MIC investigation or monitoring should be performed as soon after a sample is collected as possible to obtain results that accurately represent pipeline conditions. Historically, the ability to swiftly test samples has been one factor that has limited meaningful data collection for use in assessing pipelines for MIC, but improvements in technology have increased the availability of more types of tests and sophisticated analyses for field use where samples area collected, Eckert says.

Various testing methods are used to determine microbiological conditions, such as the species of microorganisms present in a sample, the total numbers of live bacteria, and comparisons of the population size of various abundant microorganisms. These methods include microbiological culture techniques; microscopy; measurement of bacteria-produced enzymes, such as hydrogenase, an enzyme produced by bacteria that use hydrogen as an energy source, and adenosine phosphosulfate (APS) reductase, an enzyme specifically associated with SRB; and molecular microbiological methods (MMMs), which are genetic-based testing methods. Many times, MIC is caused by the activities of several different organisms that form a community, and pipeline analysis has typically included testing for SRB, APB, general aerobic and anaerobic microorganisms, and, in some cases, iron-depositing and iron-reducing bacteria.
When using microbiological culture techniques, the objective is to approximate the size of the viable microorganism population in a solid or liquid sample using semi-quantitative estimates, or preferably, the most probable number (MPN) method.3 Although the culture technique is well established in the industry, comments Eckert, one drawback is the types of microorganisms that can be cultured in liquid media may only represent a small percentage of those present in the solid or fluid because some microorganisms, such as Archaea, are very difficult to cultivate using this method.

Microscopy is frequently used to determine the overall numbers of microorganisms present in liquid or sludge samples directly without regard to their species and has been utilized as an analysis method for many years. Epifluorescent microscopy is a technique that helps differentiate microorganisms from debris, and also can be used to examine microorganisms’ specific cellular structures. Samples are treated with a stain that fluoresces when viewed under a specific wavelength of ultraviolet (UV) light. Using energy dispersive spectroscopy (EDS) to examine samples provides information on the elemental composition of materials (e.g. scale and corrosion products) and the distribution of the constituents in the sample. Analysis with x-ray diffraction (XRD) identifies the mineralogical compounds present. For example, Eckert says, EDS would reveal that carbon and oxygen are present in the sample, while XRD would show that iron carbonate—typically a corrosion product—is there as well. Methods such as gas chromatography-mass spectrometry (GC-MS) or high-pressure liquid chromatography (HPLC) are used in the laboratory to determine if any organic acids are present in the sample that may be a byproduct of bacterial activity.

Additionally, testing for the presence of the hydrogenase, an enzyme produced by bacteria that use hydrogen as an energy source, is a method used to itemize bacteria populations in corrosion deposits and water samples in the field. Measurement of adenosine phosphosulfate (APS) reductase, an enzyme specifically associated with SRB, provides an indication of the active SRB concentration present in a bacterial sample.
Genetic methods have recently become increasingly available for detecting, quantifying, and identifying microorganisms present at corrosion sites. MMMs are culture-independent approaches that analyze samples directly by measuring the genetic material they contain. Because microorganism growth prior to testing is not required, MMMs need only very small samples, with or without live bacteria, for analysis. Once genetic materials are extracted from the sample, assays are performed in the laboratory, which render more precise quantification of various types of bacteria present than culture tests.
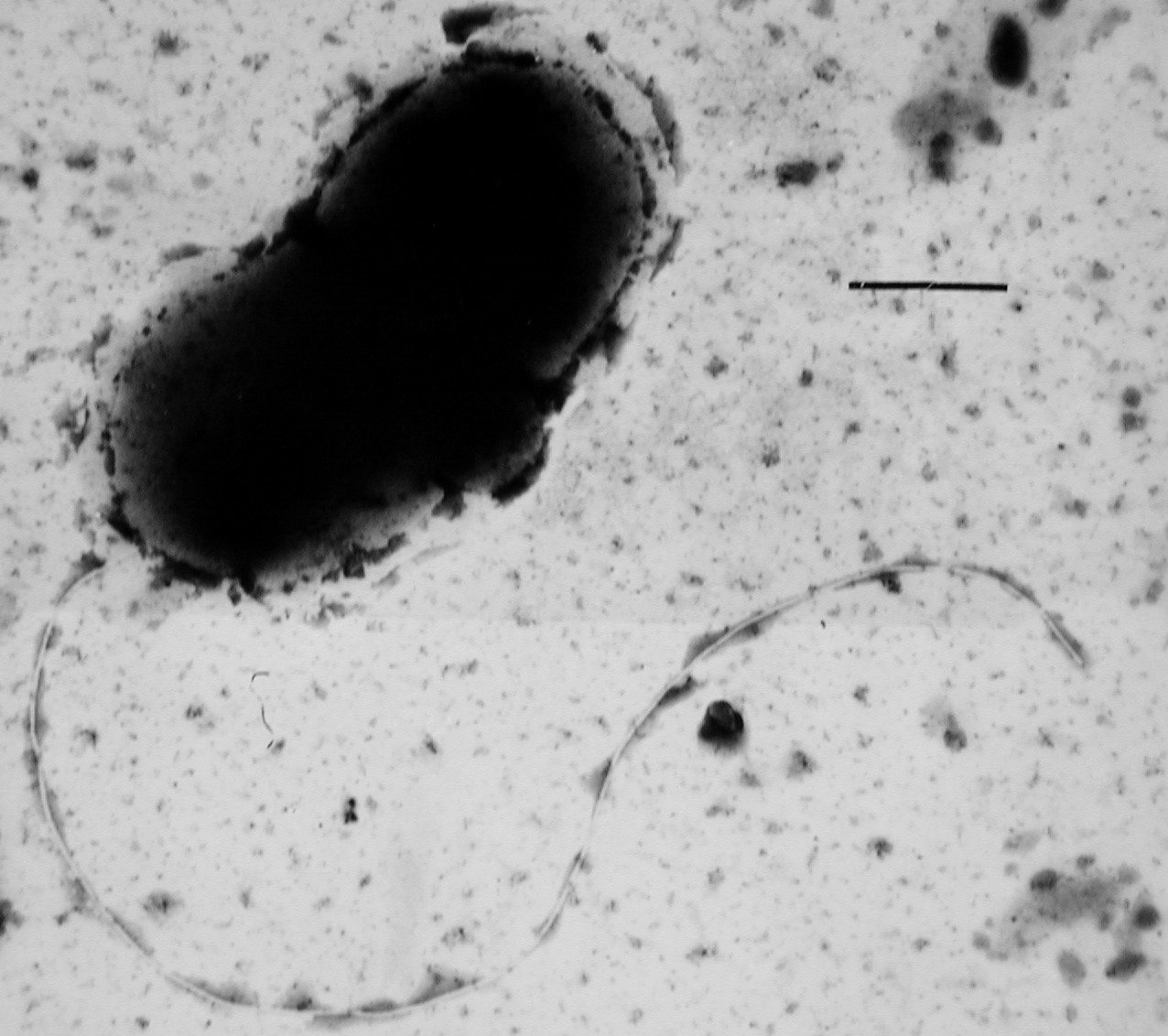
Eckert notes that two MMMs, quantitative polymerase chain reaction (qPCR) and denaturing gradient gel electrophoresis (DGGE), are often used in combination to analyze genetic samples. The qPCR method, a method that is gaining momentum for determining the identity of microorganisms in complex environmental samples, applies a modified PCR to replicate a single or several pieces of DNA across several orders of magnitude so that millions of copies of particular DNA sequences are generated. The qPCR method measures living, inactive, and dead microorganisms and may be used to count the total number of microorganisms or a specific genus/species of microorganism in nearly any type of sample, including produced fluids, oils/emulsions, and solids. This method uses synthetic DNA tagged with a fluorescent molecule to identify specific targets (microorganisms of interest [e.g., SRB]) because of their potential influence on corrosion mechanisms.
DGGE compares microbial communities across a number of different samples. With DGGE, genetic material in individual samples is amplified by PCR and subsequently compared by sorting molecules based on their reaction to an electric field (electrophoresis). This MMM is used for identifying dominant groups of microorganisms in individual samples and for evaluating how the microorganisms are distributed between samples.
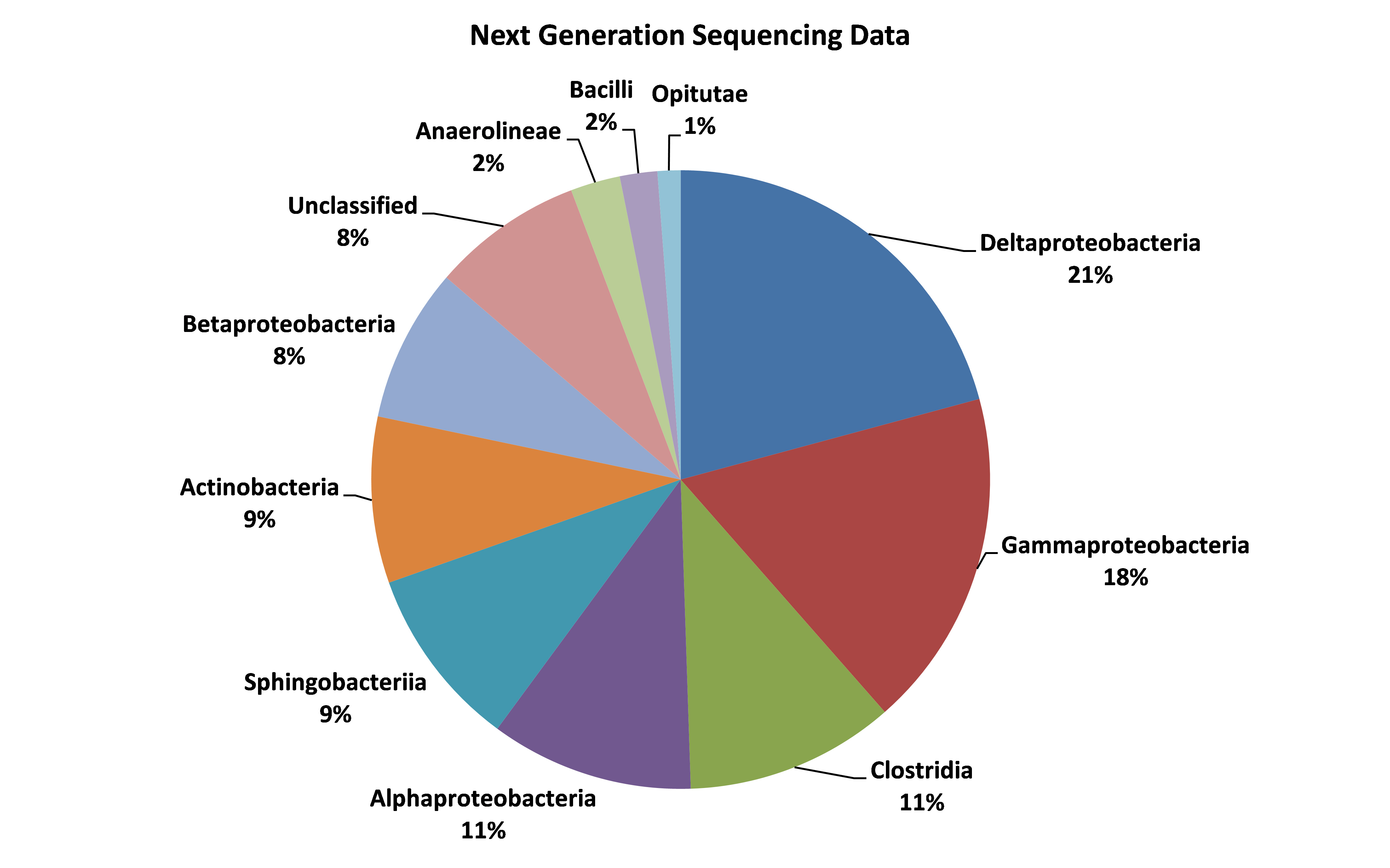
Another emerging approach, next-generation sequencing (NGS), enables identification of all the microorganisms in a sample rather than specific targeted species. The approach will not provide specific numbers of particular organisms in the sample, Eckert explains; but instead it delivers a snapshot of the diverse microbial population and how it is distributed in the sample.
Corrosion Monitoring
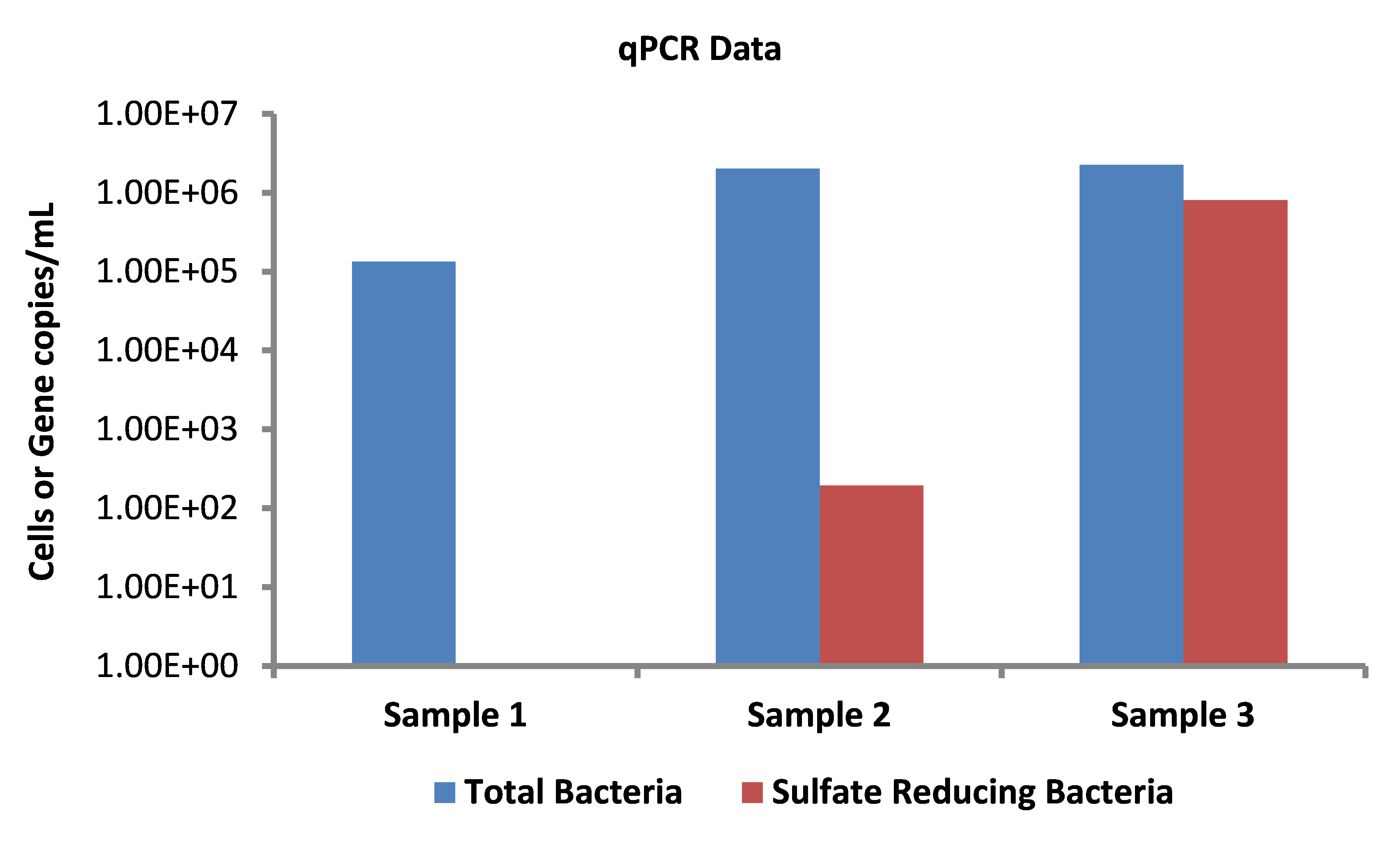 Because MIC cannot be diagnosed solely with microbiological data, the best approach to determining the presence of internal MIC in a pipeline is to integrate other applicable pipeline and corrosion data with microbiological test results. These data include operating conditions (flow rate, temperature, pressure, pigging frequency, and chemical treatment injection rates); chemical composition of the gases, liquids, and solids transported; data from corrosion coupons and probes that show the rates of corrosion, pitting, and metal loss as well as the corrosion products present; and routine inspection results. Data analysis should demonstrate that microorganisms and their activities, as opposed to abiotic mechanisms, are responsible for providing the predominant influence over the corrosion mechanism present in the pipeline.
Because MIC cannot be diagnosed solely with microbiological data, the best approach to determining the presence of internal MIC in a pipeline is to integrate other applicable pipeline and corrosion data with microbiological test results. These data include operating conditions (flow rate, temperature, pressure, pigging frequency, and chemical treatment injection rates); chemical composition of the gases, liquids, and solids transported; data from corrosion coupons and probes that show the rates of corrosion, pitting, and metal loss as well as the corrosion products present; and routine inspection results. Data analysis should demonstrate that microorganisms and their activities, as opposed to abiotic mechanisms, are responsible for providing the predominant influence over the corrosion mechanism present in the pipeline.
Monitoring the corrosiveness of pipeline contents is frequently done using coupons inserted into the pipeline at locations where corrosion is a potential threat. When possible, corrosion monitoring data and microbiological test data should be collected from the same locations. Corrosion products may be taken from the metal surface, a lining, or from a pit underneath a deposit that has been removed. When corrosion monitoring and microbiological monitoring are performed at different locations on a pipeline, the relationship between the two test locations should be identified (e.g., upstream, downstream, similar operating conditions, etc.).
 Simply reducing the numbers of viable microorganisms in a pipeline system won’t necessarily control internal corrosion in the pipeline system. A mitigation strategy typically addresses both the microbiological and corrosion threats where MIC is present. Knowing the identity and characteristics of the microorganisms present and how they interact with the pipeline system promotes an understanding of their impact on the corrosion that is being experienced, which is important when developing a mitigation strategy. A successful mitigation treatment can only be designed when the problem has been properly diagnosed. General information about methods for controlling MIC by design, operation, and specific measures, such as maintenance pigging and biocide treatment, can be found in NACE standard SP0106.4Inspection techniques commonly used to detect and monitor corrosion-related damage generally include visual inspection, ultrasonic testing (UT), radiographic testing (RT), and magnetic flux methods. The results can be used to establish the orientation, distribution, density, size, shape, and extent of internal corrosion damage. Inline inspection (ILI) may provide information about the location and severity of internal corrosion relative to operating parameters, design, elevation, and other considerations; and ILI data may be used to identify sample locations for microbiological and chemical testing of pipeline fluids. As with most data related to MIC assessment, longer-term operating parameter trends are preferred over one or a few data points as these provide more precise information.
Simply reducing the numbers of viable microorganisms in a pipeline system won’t necessarily control internal corrosion in the pipeline system. A mitigation strategy typically addresses both the microbiological and corrosion threats where MIC is present. Knowing the identity and characteristics of the microorganisms present and how they interact with the pipeline system promotes an understanding of their impact on the corrosion that is being experienced, which is important when developing a mitigation strategy. A successful mitigation treatment can only be designed when the problem has been properly diagnosed. General information about methods for controlling MIC by design, operation, and specific measures, such as maintenance pigging and biocide treatment, can be found in NACE standard SP0106.4Inspection techniques commonly used to detect and monitor corrosion-related damage generally include visual inspection, ultrasonic testing (UT), radiographic testing (RT), and magnetic flux methods. The results can be used to establish the orientation, distribution, density, size, shape, and extent of internal corrosion damage. Inline inspection (ILI) may provide information about the location and severity of internal corrosion relative to operating parameters, design, elevation, and other considerations; and ILI data may be used to identify sample locations for microbiological and chemical testing of pipeline fluids. As with most data related to MIC assessment, longer-term operating parameter trends are preferred over one or a few data points as these provide more precise information.
Editor’s note: NACE standard TM0212-2012 applies to the internal surfaces of pipelines, and describes types of microorganisms, mechanisms by which MIC occurs, methods for sampling and testing for the presence of microorganisms, research results, and interpretation of test results. It describes methodologies by which the appropriate tools and techniques may be selected and practically applied. The methods presented in this standard represent the general consensus of industry experts in pipeline corrosion and microbiology at the time this standard was published. This standard test method was prepared by TG 254, “Microbiologically Influenced Corrosion on Internal Surfaces of Pipelines: Detection, Testing, and Evaluation—Standard Test Method.” TG 254 is administered by Specific Technology Group (STG) 35, “Pipelines, Tanks, and Well Casings.” This standard is issued by NACE under the auspices of STG 35.
References
1 NACE TM0212-2012, “Detection, Testing, and Evaluation of Microbiologically
Influenced Corrosion on Internal Surfaces of Pipelines” (Houston, TX: NACE International, 2012).
2 R. Eckert, T.L. Skovhus, “Using Molecular Microbiological Methods to Investigate MIC in the Oil and Gas Industry,” MP 50, 8 (2011).
3 NACE TM0194-2014, “Field Monitoring of Bacterial Growth in Oil and Gas Systems” (Houston, TX: NACE, 2014).
4 NACE SP0106-2006, “Control of Internal Corrosion in Steel Pipelines and Piping Systems” (Houston, TX: NACE, 2006).