Corrosion and the degradation it causes to infrastructure and assets costs the Australian economy many billions of dollars annually, and is approximated to be 3 to 5% of the country’s annual gross domestic product (GDP). The estimated annual cost of corrosion to the urban water industry alone is $928 million ± 30%, according to a report commissioned by the Australasian Corrosion Association (ACA) (Kerrimuir, Victoria, Australia),1 which recorded and measured up to 117 indicators from 73 Australian water utilities serving ~75% of Australia’s population.
According to NACE International members Amy Spark and Ivan Cole with the Commonwealth Scientific and Industrial Research Organisation (CSIRO) (Clayton, Victoria, Australia), and David Law and Liam Ward with RMIT University (Melbourne, Victoria, Australia), 70% of Australia’s potable water pipeline network, including a large number of critical water transmission mains, is comprised of ferrous metals. These materials are highly susceptible to corrosion, particularly microbiologically influenced corrosion (MIC), when buried in soil. Because soil was considered to be a benign environment for many years, protecting these assets from external corrosion was thought to be unnecessary. Localized corrosion of the external pipe surface, however, has been found to be a leading contributor to water pipe leaks and failures; and one of the primary mechanisms that cause localized pitting corrosion in potable water networks is MIC. This is due to the action of biofilms that form on the exterior surfaces of pipes.
In a paper2 presented at CORROSION 2016 in Vancouver, British Columbia, Canada, Spark, Cole, Law, and Ward discuss a new technique developed to study MIC of metal in soil. Rather than relying on the aqueous solutions traditionally used, this novel test method utilizes agar (a gel-like substance derived from algae that is used for culturing bacteria) to more closely simulate both the physical structure and chemical components of soil. They explain that the types of nutrients present in an environment or in a laboratory growth medium are important when determining the likelihood and extent of corrosion due to specific microbial species. Some nutrients have been shown to have an inhibitory effect, although this depends on the type of nutrients and the ratio of aggressive ions (e.g., chlorides) to the inhibitory compounds present. Changes in corrosion rate when bacteria are present could be due to the indirect effect of the bacteria consuming nutrients and changing the balance between aggressive and inhibitory components of the environment or medium.
The authors comment that many different types of bacteria—including sulfur-reducing bacteria, acid-producing bacteria, sulfur-oxidizing bacteria, iron-reducing bacteria, and several other groups of microbes—have been associated with MIC of carbon steel (CS), copper, and stainless steel. They note that the number and type of microbial species present, as well as the environment, play a crucial role in localized corrosion. Biofilms with a mixture of microbe species form naturally on many metal surfaces, and it is these biofilms that are most likely to be linked to increased localized corrosion.
The new methodology being developed involves an electrochemical test conducted on CS coupons (either with or without the presence of bacteria) exposed to semi-solid agar electrolyte mixture comprised of a low concentration of sodium chloride (NaCl) and a peptide-based nutrient broth. Pseudomonas fluorescens, common rod-shaped bacteria that colonize soil, water, and plant surface environments and are highly likely to be present in the conditions around a pipe, are used in the test. These bacteria have been previously implicated as contributing to localized corrosion of CS. Microbiological-grade agar is used to simulate both the physical structure and chemical components of soil more closely than can be done with aqueous solutions, which can be made to mimic the chemical composition of soil, the authors say, but do not take into account the physical structure of soil, which plays a major role in electrochemical interactions between soil, microbes, and the steel.
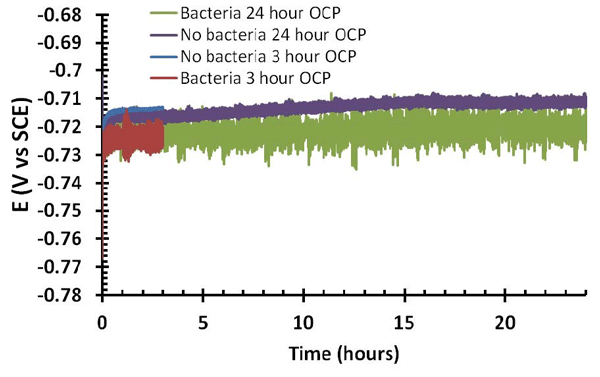
For the tests conducted by the authors, sterilized CS coupons were swabbed with a P. fluorescens culture. A standard three-electrode polarization cell was constructed with a 500-mm2 platinized titanium mesh counter electrode, a standard calomel reference electrode (SCE), and a polished CS sample (either with or without bacteria swabbed onto the surface) as the working electrode. An agar electrolyte solution (4 g/L of agar, 12.5 g/L of nutrient broth, and 2.5 g/L of NaCl in distilled water) was poured into the completed cell and then allowed to set for ~2.5 h. The open circuit potential (OCP) was then measured for durations of 3 and 24 h.
“Because we were working with bacteria, we had to maintain a sterile environment until we added our bacteria to the system,” says Spark, noting that the environment needed to remain aseptic so the authors knew the effects they were seeing were due to the bacteria they had introduced and not something from the air in the lab. “We also had to be much more careful with cooling the agar before we added it to the system so that the bacteria didn't go through thermal shock, which would kill them off before they could do anything to our metal,” she adds.
After the OCP measurements were taken, cyclic potentiodynamic scans were done at a scan rate between -0.4V and 0.4V vs. SCE to determine how the presence of bacteria contributed to corrosion. Tafel plots based on these scans were used to determine the corrosion potential and corrosion currents for each experiment. The samples were then removed from the polarization cell, treated, dried, and made ready for viewing using scanning electron microscopy (SEM).
These initial results suggest that under the test conditions, P. fluorescens increased the corrosion of the steel. For samples with and without bacteria, a positive shift in the OCP measurements was seen over the 24 h test time, which suggests the buildup of a protective film on the steel due to the peptide-based nutrients in the agar electrolyte mixture. According to the authors, however, the bacteria had two key effects on the OCP. They made the overall potential values more negative than those for samples without bacteria, and they also increased the variability of the OCP measurements from one point to another. The more negative OCP measurements with the addition of bacteria to the system imply that the bacteria were potentially interfering with the development of the protective film. The samples with bacteria also had more negative corrosion potential readings and higher corrosion currents.
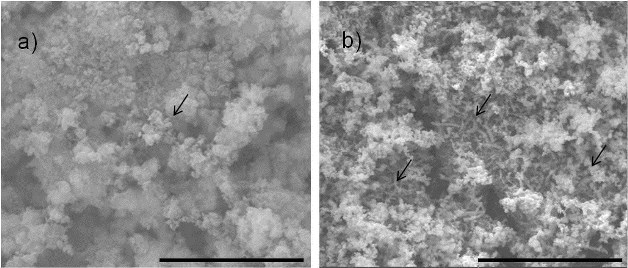
SEM imaging of the surfaces of samples with bacteria illustrated different protective film morphologies between the samples that underwent a 3-h relaxed potential hold period and those that underwent a 24-h relaxed potential hold period. The 3-h samples showed very few obviously present bacteria cells on the surface, with only one being clearly identified on the surface area examined with SEM. The corrosion product on the surface also appeared to be less dense. On the 24-h samples, more bacterial cells were visible on the surface, and the oxide film itself was denser and not dominated by corrosion products.
The authors explain that the longer free potential hold time led to a decrease in the electrochemical drive that causes corrosion to occur, and propose that with the increased hold time, a greater buildup of nutrients was possible, and that these nutrients formed a protective layer on the steel surface that had an inhibitive effect. The addition of bacteria to the system, however, decreased electrochemical stability that was intensified with the longer free potential hold. This suggested that the longer a biofilm has to form, the more it competitively interacts with the oxide/nutrient film forming on the steel surface.
“Going in we weren't sure what effect the bacteria would have, as there were very few studies conducted with our species of choice,” Spark comments. “In some earlier work we had shown that the peptide nutrients we were using had an inhibitive effect on corrosion, so we weren't surprised that with extended time for a film to form before accelerated corrosion testing, there were indications of this with and without bacteria. This also helped us to understand why the effect of the bacteria was not as large as we might have expected looking at studies with other bacterial species.”
With adaptation, the authors say, this system could be used to accurately replicate corrosion in a soil environment with the introduction of nutrients to match the soil environment in question and cultured biofilms found along buried pipelines. “We are hoping this research will enable more information to be gathered about the mechanisms of localized corrosion and how it occurs and how bacteria and other microbes interact with the environment and the metals buried in soil. Now that we have a suitable analogue for soil in the laboratory, we can investigate many different parameters for this that can then be used to inform policies for coatings, materials, and the initial sites chosen for pipelines as well as policy around the methods for monitoring, repairing, and replacing pipelines that have been installed,” Spark says.
References
1 G. Moore, “Corrosion Challenges—Urban Water Industry,” Corrosion Challenges Project 2010, Australasian Corrosion Association, 2010.
2 A. Spark, et al., “MIC Studies of Buried Potable Water Pipelines using Semi-solid Agar as an Analogue for Soil,” CORROSION 2016 paper no. 7850 (Houston, TX: NACE International, 2016).