In 1928, physician and microbiologist Alexander Fleming returned to London from a summer vacation in Scotland to find that a mold called Penicillium notatum had contaminated his Petri dishes and that it prevented the normal growth of staph bacteria. His discovery of penicillin was a happy accident that changed modern medicine.
Discoveries and inventions such as insulin, the microwave oven, and even the children’s toy called the Slinky were among other happy accidents that have led to many practical and useful discoveries through the years.
Now Marco D’Elia and his research colleagues hope to introduce to the world another such accidental discovery—an anti-corrosion polymer that is self-healing.
About PPM
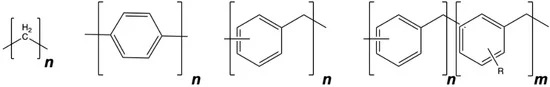
Poly(phenylene methylene), or PPM, is a multifunctional polymer featuring hydrophobicity, high thermal stability, fluorescence, and thermoplastic processability, the researchers explain in one of their scientific papers. The research team at ETH Zurich (Federal Institute of Technology Zurich) developed the plastic and believe it could improve and simplify corrosion protection.
Markus Niederberger, chair of the Laboratory for Multifunctional Materials at ETH Zurich and Professor Walter Caseri led the research, which preceded D’Elia’s arrival.
D’Elia, a native of Como, Italy, received his master’s in Milan while studying PPM. He then earned his doctorate from ETH Zurich continuing his work with PPM and now is in a fellowship called Deep-Tech Pioneers at the university to bring the substance to market.
“We started to find this strange behavior in which the coating was not always failing in the correct way,” says D’Elia, who is doing his post-doctoral work on the substance. “We found out that after being damaged, the coating started to restore its performance to what it was before the damage.”
Other contributing researchers were Mirko Magni, Thomas Romanò, and Stefano P.M. Trasatti of the Department of Environmental Science and Policy at the University of Milan in Italy.
“We learned that the polymer had a good chance to be a good coating just relying on the fact that it was a thermoplastic material, it was fluorescent, and it was highly thermal stable,” D’Elia says. “These material properties were suggesting that this was good material for anti-corrosion, but we did not know about the self-healing at the beginning. It was a bit of serendipity.”
The researchers initially applied PPM by hot-pressing it onto aluminum alloy AA2024, testing its protection properties and evaluating the proper thickness required, which is on the order of tens of micrometers.
PPM is structurally located between polyethylene and polyphenylene, and its fluorescence facilitates detection of failures in the coating upon corrosion, the authors write in “Smart Anticorrosion Coatings Based on Poly(phenylene methylene): An Assessment of the Intrinsic Self-Healing Behavior of the Copolymer,” published in MDPI Polymers in August 2022.
Coatings made of PPM alone tend to crack, a drawback that can be overcome with the addition of plasticizers or by using PPM copolymers with alkoxy side chains along the polymer backbone, the researchers write. These additions can yield continuous films that can be used as anti-corrosion coatings.
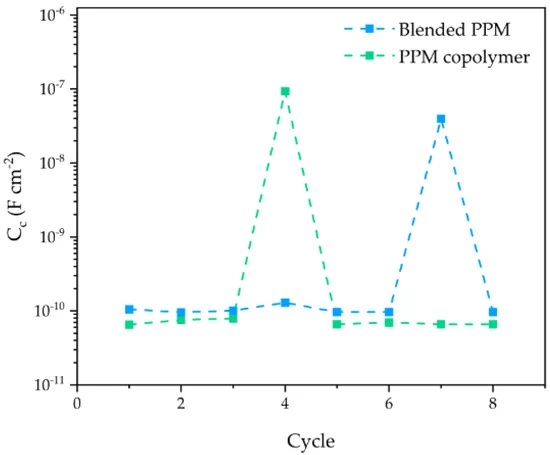
The scientists investigated the corrosion protection ability of the described polymer coatings by means of the accelerated cyclic electrochemical technique (ACET).
“At the end of the ACET test, the blended coating clearly evidences at least one hole in the protective coating, responsible for the occurrence of a localized corrosion event at the surface of the exposed AA2024 (occurring during the relaxation step of each ACET cycle) and for the increased cathodic current flowing during the last polarization step (i.e., wider active surface, higher current),” the authors write.
Testing a Controlled Defect
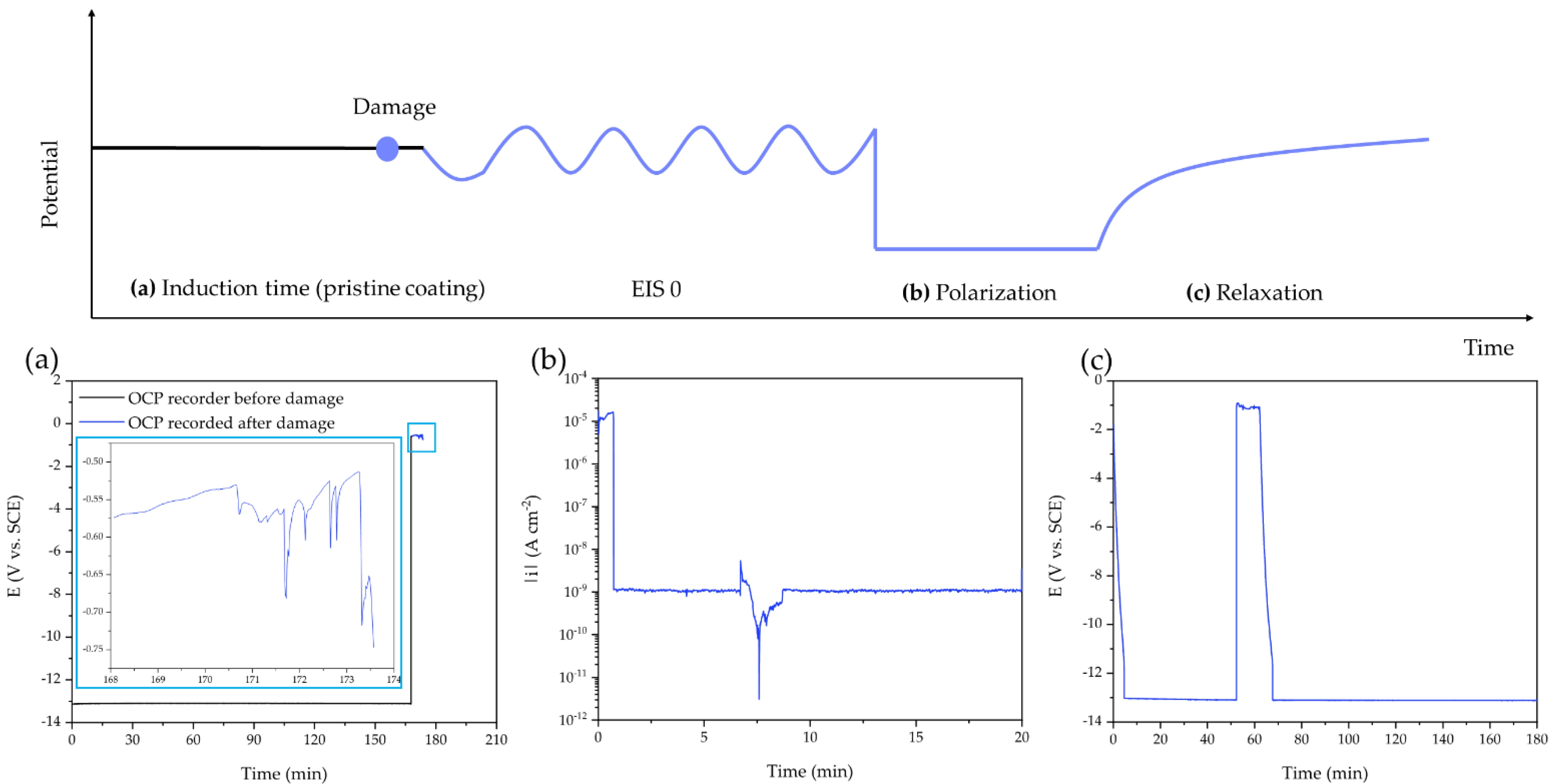
These results prompted the researchers to further investigate the self-healing properties of the polymer by introducing a controlled defect in the coating by exposing it to synthetic sea water and applying an artificial scratch to force a direct physical and electric contact between the solution and the metal surface.
“After the artificial scratch was applied, a sudden drop of the OCP (open circuit potential) at around −0.5 V and −0.7 V (vs. SCE) was detected. These values are compatible with naked aluminum alloys in chloride solution,” the authors continue. “During the cathodic polarization step, the current density decreases by about four orders of magnitude in the first few minutes. This drop means that the coating quickly established a more protecting feature, attributable to the self-healing of the artificial crack.”
The evidence that self-healing takes place during both cathodic polarization and the relaxation step suggests that the self-healing mechanism can be triggered by thermal shocks, the researchers write.
“Corrosion is a very exothermic reaction,” D’Elia says. “Heat melts the polymer, and the polymer flows into the hole and that’s why it quenches the corrosion attack.”
Fluorescence, Recyclability
D’Elia explains that PPM’s fluorescence is another trait that did not seem desirable at first but turned out to be desirable.
“Fluorescence is an added value for us because, for example, you can be sure if you think pipelines have water inside,” he says. “If you have a coating that is fluorescent, you can go in with a camera in UV light and then you will see the corrosion spots.”
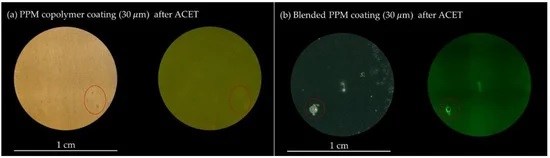
A black spot visually identifies the defect in the polymer coating when irradiated with UV light because of the lack of green fluorescence emission coming from the blended PPM layer around it.
“The occurrence of the damage in the PPM coating is also associated with an intensification of the fluorescence emission just around the hole, a further useful diagnostic tool in identifying even small breaks in the coating,” they write.
Another important aspect of PPM is that it cures in minutes and that it is recyclable, D’Elia says. Workers can completely remove and recycle PPM with little material loss, and the recycled polymer can then be applied to another surface with no loss to its special properties and functions.
Practical Applications
He is in the midst of an 18-month period of product development testing PPM’s application in such varied but industrially relevant environments as electronics, marine applications, and in aircraft, where materials need to be lightweight but strong. Another advantage of PPM is that pretreatment is not required before it is applied, which could save time as well as money.
“The reaction is very scalable, and the raw material demand is cheaper than other monomers present in the market right now,” D’Elia says, adding that his dream is to see PPM used to protect the flood barrier walls around the city of Venice, Italy.
The authors conclude their scientific paper by saying that PPM’s encouraging corrosion protection ability, thermoplastic behavior, and self-healing ability under corrosion attack lead them to believe that this type of material “represents a promising thermoplastic alternative to the thermosetting resins commonly used for metallic corrosion protection.”
Editor’s note: This article first appeared in the March 2024 print issue of Materials Performance (MP) Magazine. Reprinted with permission.
