The Arvida Bridge is located ~190 km (120 mi) north of Quebec City, Canada. Built by the Dominion Bridge Co. and completed in 1950, it was the world’s first long-span aluminum road bridge. The bridge spans the Saguenay River as it rushes through a rocky ravine (Figure 1). A large aluminum smelter, which was owned by the Aluminum Co. of Canada (Alcan) and is now operated by Rio Tinto Alcan, is located ~5 km (3 mi) east of the bridge.
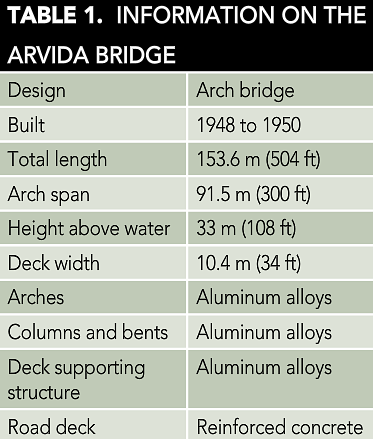 After World War II, Alcan wanted to expand the peacetime uses of aluminum, and building a road bridge seemed like a fitting expression of the company’s pioneering spirit. Information on the bridge is summarized in Table 1.
After World War II, Alcan wanted to expand the peacetime uses of aluminum, and building a road bridge seemed like a fitting expression of the company’s pioneering spirit. Information on the bridge is summarized in Table 1.
Materials of Construction
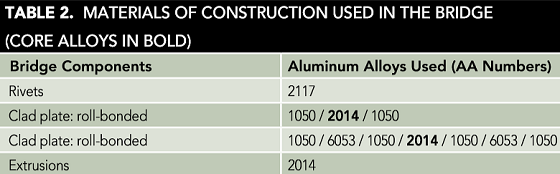
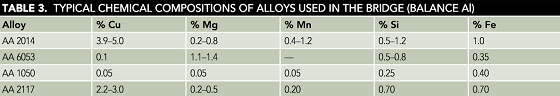
Table 2 lists the principal components of the bridge along with their Aluminum Association (AA) alloy designation numbers. Table 3 gives the typical chemical compositions of those alloys.
The Environment
The Saguenay River region experiences long (four- to five-month) winters with lots of ice and snow. The bridge is located in a largely rural area and, for most of the year, is upwind of the aluminum smelter. As the river rushes through the ravine, it often creates a fine mist. Following an accident many winters ago, the local authority started applying deicing salt to the approach roads. Some of this salt undoubtedly gets tracked onto the bridge by vehicles.
Bridge Inspection in 1984
By 1984, local officials were aware that some structural components had suffered corrosion damage. The author (then working in the Corrosion Group of Alcan International, Ltd.) was asked to take part in a bridge inspection and to make suggestions for repair work.
Since permits were not issued to climb out onto the arches, the inspection concentrated on the support columns (sometimes called piers) and the bents on each bank of the ravine. Having a “Double-H” shape, the bents extend from the concrete bridge abutments up to the road deck. They were designed to carry lateral as well as vertical loads. The columns, bents, and arches had been made by riveting plates of aluminum together to form four-sided, hollow box sections.
Support Columns
Except for the bottom 150 to 200 mm (6 to 8 in), the north and south columns were found to be in good condition. All the columns (eight on each river bank) had corrosion damage at their bases. The damage varied from some delamination to severe exfoliation of the external and internal surfaces (Figure 2). Over the years, leaves, grass, and dirt had entered the box-sections via the hand-holes and had collected at the bottom of the columns. This debris was partially blocking the semicircular drainage holes and was forming a damp poultice up against the steel base-plates and the feet of the aluminum columns. Samples of the debris were taken for laboratory analysis.

The corrosion damage appeared to have initiated at the cut edges of the aluminum plates, especially where those edges were in direct contact with the steel base plates. This type of deterioration (to varying degrees of severity) was observed at the bases of all 16 columns. A loose piece of aluminum was detached from one column and taken for laboratory analysis.
North and South Bents
The bents and their steel anchor bolts were found to be in good condition. A few small areas of exfoliation or delamination were noticed on the bottom horizontal cross-member of the north bent. The bent on the south bank did not appear to have suffered any corrosion damage.
Arches
As viewed with high-power binoculars from the bridge abutments, the arches appeared to be in good condition and no external delamination or exfoliation was observed. However, some of the cross-braces between the arches were observed to have suffered corrosion damage.
Road Deck and Expansion Joints
Small pieces of concrete had broken away from under the road deck and the west sidewalk, thus exposing the steel reinforcing bars. Corrosion damage to the steel rebar did not appear to be too serious at that time.
Some of the girders/beams that support the road deck were observed to have areas of corrosion damage—especially around the expansion joints where water drains down through the joints from the deck. White deposits were taken from the aluminum structural members near the north expansion joint for laboratory analysis.
Laboratory Investigations
Chemical analysis identified the presence of soluble chlorides in the “poultice” samples collected from the column bases. The white deposits taken near the north expansion joint were also found to contain traces of soluble chlorides. It seems very likely that the chlorides originated from deicing salt applied to the approach roads.

The loose piece of aluminum taken from one of the columns was mounted in plastic, polished, etched, and subjected to microscopic examination and analysis. It was found to consist of laminations of Alloys AA 2014, AA 6053, and AA 1050. The corrosion damage is shown in Figure 3 and is typical of exfoliation corrosion, whereby the original metal is converted into a series of layers of corroded metal and corrosion products.
Exfoliation Corrosion
Not all aluminum alloys or alloy tempers are susceptible to exfoliation corrosion. It is most common in Al–Cu alloys (2xxx series) and can occur in some Al–Mg (5xxx series), and Al–Mg–Si (6xxx series) alloys. It occurs in alloys with markedly directional microstructures (e.g., where alloy processing has created elongated, “pancake” grains [crystals]). The attack proceeds along narrow, selective sub-surface paths that are parallel to the main surface. Voluminous corrosion products start to force the layers apart and flakes of metal can peel away from the surface. At later stages, the damage resembles the pages of a partly opened book. Exfoliation corrosion has often been observed at sheared edges and when the aluminum is coupled to a more noble metal.1-2
On a microstructural level, it has been suggested that exfoliation corrosion is due to: (i) aligned intergranular precipitates, (ii) subgrain boundary precipitates, or (iii) aligned strata with slightly different compositions. Exfoliation corrosion can often be eliminated by changing alloy processing temperatures/times to achieve more uniform precipitation within grains or a more advanced stage of precipitation.2
Suggestions for Repairs
Following the 1984 bridge inspection, suggestions for repair work focused on the damage at the bases of the 16 columns. One suggestion involved burying the corroded aluminum in new concrete. Given the porous nature of concrete and the risk of poor adhesion between the concrete and aluminum, this idea was rejected for not providing a long-lasting repair.
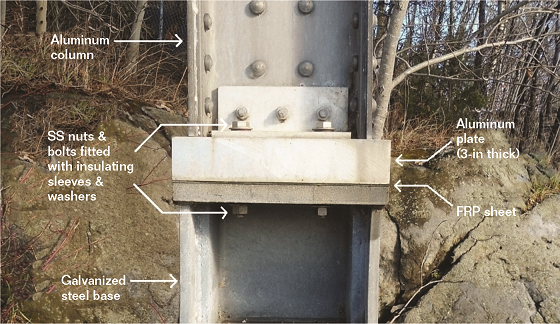
Another suggestion involved cutting away the base portions of the columns and replacing them with steel bases. A sheet of electrical insulation material would be placed between the steel base and the aluminum column to prevent galvanic corrosion. In addition, the nuts and bolts holding the column to the base would be fitted with insulating washers and sleeves to eliminate electrical connections. This repair design was chosen with the following improvements: (i) the steel bases (fabricated from heavy plates) were hot-dip galvanized, and (ii) a thick plate of aluminum was inserted between the column base and the sheet of insulation material (to spread the load and prevent puncturing of the insulation).
Repair Work in 1985-1986
The columns were repaired one at a time while the bridge remained open. Traffic was reduced to one lane on the side of the bridge opposite to the column under repair. Each column was supported by a jacking system during the cutting and installation of the new base sections. Cuts were made just above the lower hand-holes. Figure 4 shows the base of one of the repaired columns. Four new drainage holes were cut at the base of each column. The heavy aluminum plates (76mm [3in] thick) are made of Alloy AA 6061-T51. The sheets of insulation material are made of fiber-reinforced plastic (FRP) and are 3mm (1/8in) thick.
L-shaped aluminum angles were attached to the column bases using aluminum alloy nuts and bolts. The angles were then secured to the new steel bases with bolts, nuts, and washers made of 18/8 stainless steel (SS). To further prevent galvanic corrosion, insulating FRP washers and sleeves were installed under and around the SS fasteners.
Recent Inspection
After 30 years in service, the repair work of 1985-1986 was found to be in very good condition. No significant rusting was observed on the galvanized steel bases (Figure 4). Only a very small area of exfoliation was found at the base of one aluminum column on the north bank.
Other repair work (not involving the columns) took place during the period May 2013 to August 2014. Some of the aluminum cross-bracing on the arches was replaced. Aluminum girders/beams under the road deck were replaced (they are made of AA 6061-T6 and 6061-T651). The concrete road deck and sidewalks were renewed, and improvements were made to the bridge bearings, the expansion joints, and to the drainage systems under the joints. Further attention was given to directing rain and melt water away from the bridge on the south side.
Conclusions
In the late 1940s, the Arvida Bridge was a courageous project to demonstrate the long-term performance of aluminum in a major civil engineering structure. General conclusions that can be drawn from the nearly 70-year lifetime of this structure are:
1. Contact between aluminum and carbon steel must be avoided.
2. Use of AA 2xxx series alloys should be minimized or avoided.
3. Prior to use, aluminum components should be sampled and tested according to ASTM G1123 to check their resistance to exfoliation corrosion.
4. The use of deicing salt on or near the bridge should be minimized
5. Salty water runoff from approach roads should be carefully directed away from the bridge.
6. Drainage from the road deck and expansion joints should be carefully collected and piped away from the bridge.
Since the 1940s, much has been learned about maximizing the corrosion resistance of aluminum alloys. Hence, selecting aluminum alloys (without cladding) that possess a good combination of strength, ease of fabrication, and corrosion resistance would be easier today. For example, AA 6061 (T6 or T651) would be expected to perform well in bridge service in mild environments. Seacoast, heavy industrial, and urban snow-belt environments may prove more challenging.
A definitive opinion on the economic performance of the Arvida Bridge over its ~70-year life cannot be offered without full knowledge of all maintenance and repair costs. Given such information, it would be interesting to compare the life-cycle cost of the aluminum bridge with that of a steel arch bridge of similar design and size and in a similar environment. A steel bridge would also have required maintenance over the same lifetime (e.g., repainting)—and some severely corroded steel components may have required costly replacement.
Acknowledgments
The author acknowledges the participation of S.C. Hedrei, E. Hay, and S. Gooden in the 1984-1986 inspection and repair work. Information about the 2013-2014 repair work was kindly supplied by P. Girard-Bélanger of the Arrondisement de Jonquière.
References
1 H.P. Godard, et al., The Corrosion of Light Metals (New York, NY: John Wiley & Sons, Inc., 1967), pp. 72-73.
2 Metals Handbook, 9th Ed., Vol. 13: Corrosion, “Corrosion of Aluminum and Aluminum Alloys” (Metals Park, OH: ASM International, 1987), pp. 594-595.